Looking for a student learning guide? It’s linked in the main menu for your course. Use the “Courses” menu above.
1. Introduction
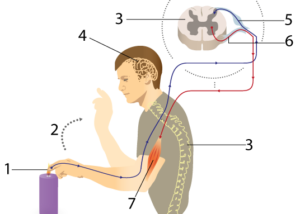
Let’s start this unit about how organisms respond to the environment by thinking about a reflex. You accidentally touch something that’s burning hot and, in an instant, you pulled your hand away from the source of the injury.
A reflex is an example of a behavior: “anything that an organism does in response to a stimulus.” In a reflex arc, the connection between stimulus and response can be reduced to a few nerves, bones, and muscles. In this module, we’ll look at more complex behaviors, and see how we can explain their underlying biology.
2. Fixed Action Patterns
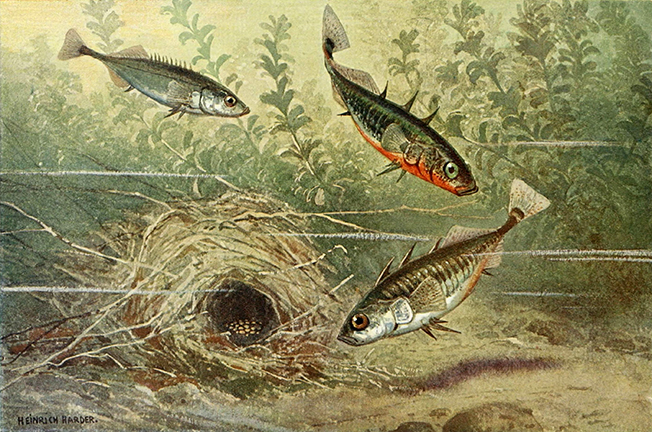
Leipzig :Quelle & Meyer,1913 (illustration 4)
The three-spined stickleback (Gasterosteus aculeatus) is a widely dispersed fish found in inland coastal waters throughout the northern hemisphere. During their mating season, stickleback males move into shallow areas where they build a tunnel-shaped nest. Once the nest is complete, the males try to attract egg-bearing females with a zigzag dance, the goal of which is to get the female to lay her eggs within the nest. The male follows the female’s egg-laying by fertilizing the eggs. Subsequently, the male will chase away any other male who approaches the nest, making sure that all the eggs within the nest are his progeny.
Stickleback behavior was studied during the middle of the last century by Nikolass Tinbergen, one of the founders of the modern study of animal behavior.
[qwiz]
[h]Fixed Action Patterns
[q]Tinbergen was interested in what type of stimulus would motivate a male stickleback to respond with aggressive behavior. To test this, Tinbergen created a variety of plastic models and presented them to a male stickleback. Based on the description of the stickleback’s life history and the picture above, predict which of the models below elicited the weakest response.
[textentry single_char=”true”]
[c]MQ ==[Qq]
[f]WWVzLiBNYWxlcyBpZGVudGlmeSByaXZhbCBtYWxlcyBieSB0aGVpciBjb2xvcmF0aW9uOiBhbGwgYnJlZWRpbmcgbWFsZXMgaGF2ZSBhIGJyaWdodCByZWQgdGhyb2F0LiBTbyBldmVuIHRob3VnaCBhIHNoYXBlIG1heSBiZSB2ZXJ5IHVubGlrZSBhIGZpc2gsIHRoZSByZWQgY29sb3Jpbmcgb24gdGhlIGJvdHRvbSBpcyB3aGF0IGVsaWNpdHMgdGhlIHJlc3BvbnNlLiDigJwxLOKAnSB3aXRob3V0IGFueSByZWQgYmVsb3csIGlzIGlnbm9yZWQu
Cg==Cg==[Qq]Continue reading below.
[c]Kg==[Qq]
[f]Tm8uIEhlcmUmIzgyMTc7cyBhIGhpbnQuIE1hbGVzIGlkZW50aWZ5IHJpdmFsIG1hbGVzIGJ5IHRoZWlyIGNvbG9yYXRpb246IGFsbCBicmVlZGluZyBtYWxlcyBoYXZlIGEgYnJpZ2h0IHJlZCB0aHJvYXQu
Cg==Cg==[Qq]Continue reading below.
[/qwiz]
For a male stickleback, any object with a red shape on the bottom acts as a key stimulus (also called a sign stimulus) that releases a fixed action pattern: an “instinctive behavioral sequence that is relatively invariant, and once initiated, inevitably runs to completion.” (Wikipedia). Fixed action patterns are highly stereotyped: they’re carried out the same way by all members of the species in which they occur, and they arise without any previous learning.

Here’s another fixed action pattern. Gull chicks are fed by both parents. As soon as a gull chick hatches from its egg, it pecks at whatever bill is nearby. Pecking, in turn, elicits the parent to regurgitate food into their chick’s mouth.
But what’s the key stimulus that elicits the pecking response? To test this, Tinbergen created a variety of cardboard models, presented them to the gull chicks, and recorded the intensity of the pecking response. Take a good look at the picture of the gull on the right, and then complete the exercise below.
[qwiz qrecord_id=”sciencemusicvideosMeister1961-Fixed action patterns and sign stimuli in gulls”]
[h]Fixed action patterns and sign stimuli in gulls
[q labels = “top”]Match the models below (each of which Tinbergen presented multiple times to gull chicks) and drag the “relative pecking response” above to the right position. The higher the number, the more intense the response.
[l]35
[fx] No, that’s not correct. Please try again.
[f*] Great!
[l]92
[fx] No. Please try again.
[f*] Good!
[l]100
[fx] No, that’s not correct. Please try again.
[f*] Excellent!
[l]125
[fx] No. Please try again.
[f*] Great!
[q]The chick’s nervous system is wired to peck at the red dot that’s close to the end of its parent’s bill. But the red stick with contrasting bars elicits an even [hangman] response.
[c]c3Ryb25nZXI=[Qq]
[q]The red stick with contrasting bars is an example of a supernormal stimulus: an exaggerated version of a stimulus to which there is an existing response tendency or any stimulus that elicits a response more strongly than the stimulus for which it evolved. (source: Wikipedia)
Read on.
[/qwiz]
Fixed action patterns are an evolutionary adaptation to ensure that a particular stimulus elicits an adaptive response. The male stickleback who’s programmed to aggressively chase away rivals will ensure that the eggs in his nest are his eggs (as opposed to being those of another male) and that they’ll safely develop into young fish that will have a chance of passing on his genes (as opposed to being eaten by a rival). The gull chick that pecks at a red spot increases its chance of getting food and surviving to adulthood, and the adult that responds ensures that his or her offspring get the food they need to survive.
But what these fixed action patterns gain in efficiency and automaticity, then lose in terms of discrimination. Once they start, they continue to completion. As a result, they can be stimulated by stimuli that wind up eliciting maladaptive behavior.
You can see this in work in the video below, which shows how a goose will return just about any shaped object back to her next. The stimulus is “something out of the next.” The fixed action pattern is “return it to the nest.”

Because fixed action patterns elicit an automatic response, they can be exploited by other species. One example of this is demonstrated by cuckoos, which are nest parasites. The cuckoo lays its egg in the nests of other birds, such as that of the reed warbler shown at left. The red gaping mouth of the cuckoo chick acts as a supernormal stimulus to the reed warbler, which feeds the cuckoo, even at the expense of providing food to her own chicks.
3. Fixed Action Patterns, Checking Understanding
[c]IGJlaGF2aW9y[Qq]
[q] In a male stickleback, every shape except for shape number “1” below acts as a [hangman] [hangman] that elicits a(n) [hangman] action [hangman].
[c]IGtleQ==[Qq]
[c]IHN0aW11bHVz[Qq]
[c]IGZpeGVk[Qq]
[c]IHBhdHRlcm4=[Qq]
[q] Fixed action patterns are highly [hangman] at eliciting a behavioral response.
[c]IGVmZmljaWVudA==
[q] Which letter below is the supernormal stimulus?
[textentry single_char=”true”]
[c]IG Q=
[f]IEV4Y2VsbGVudC4gJiM4MjIwO0QmIzgyMjE7IGlzIHRoZSBzdXBlcm5vcm1hbCBzdGltdWx1cy4=
Cg==[Qq]
[c]IEVudGVyIHRoZSB3b3Jk[Qq]
[c]ICo=[Qq]
[f]IE5vLiBUaGUgc3VwZXJub3JtYWwgc3RpbXVsdXMgaXMgdGhlIG9uZSB0aGF0IGVsaWNpdHMgdGhlIHN0cm9uZ2VzdCByZXNwb25zZS4=
Cg==[Qq]
[q] For a gull chick, the red stick with white bars near its tip acts as a [hangman] stimulus.
[c]IHN1cGVybm9ybWFs[Qq]
[q] The image below is an example of nest [hangman]. The cuckoo has evolved to exploit a(n) [hangman] action [hangman] in the reed warbler. The cuckoo’s gaping mouth is an example of a [hangman] stimulus, essentially tricking the reed warbler into feeding the cuckoo chick, even at the expense of its own offspring.
[c]IHBhcmFzaXRpc20=[Qq]
[c]Zml4ZWQ=[Qq]
[c]cGF0dGVybg==[Qq]
[c]a2V5[Qq]
[/qwiz]
4. Behavior can have a genetic basis, and can evolve over time
Fixed action patterns can be thought of as complex reflexes, elicited by a key stimulus. Reflexes are rooted in the organization of nerves, muscles, and other structures. Since genes underlie the developmental patterns that give rise to these structures, it’s not a big leap to suggest that genes underlie behavior as well. Here’s some of the evidence for that claim.
4a. Genes influencing Circadian Rhythms in Drosophila
The per gene plays a key role in circadian rhythms. Circadian rhythms are biological processes that oscillate around a 24-hour time period. The key fact about these rhythms is that they’re internally driven: even in the absence of environmental cues (such as the dawning of a new day), they continue in a cycle that’s about 24 hours long.
Circadian rhythms are the reason why travelers who fly from one time zone to another experience jet lag. The traveler’s circadian rhythms, which are in sync with their previous time zone, cause them to get tired and wake up at the wrong time (relative to the new time zone they’ve flown into). After a few days, however, their circadian rhythms become entrained to the new time zone.
In the fruit fly Drosophila, the per gene influences eclosion (emergence from a cocoon) and locomotor activity (any activity that moves the organism from one location to another). In flies with the wild-type per allele, the expression of the gene leads to activity that’s close to a 24-hour cycle. In other words, even when these fly larvae are kept in the dark, their activity patterns remain about 24 hours long.
Two mutant versions of this gene alter this cycle to shorten it or lengthen it: pers leads to a 19-hour oscillation in locomotor activity and early eclosion; perL leads to a 29-hour oscillation and late eclosion.
Homologous versions of these alleles have been found in mammals (including humans). The exercise below shows the effect of a circadian rhythm gene found in mice.
[qwiz]
[h]Genes controlling Circadian Rhythms in mice
[q]The diagram below shows two actograms for two strains of mice. Actograms are graphic representations of an animal’s activity. A dark bar indicates a period of activity. The X-axis represents a 24-hour day. The Y axis shows a series of 17 days during which the mice’s activity was recorded. During the first week of the study, the mice were kept in 12 hours of light and 12 hours of darkness. After day 7, the mice were kept in continuous darkness.
One of these graphs shows the effect of a mutation in a circadian rhythm gene. Which one?
[textentry single_char=”true”]
[c]IG I=[Qq]
[f]IEV4Y2VsbGVudC4gJiM4MjIwO0ImIzgyMjE7IGlzIGFuIGFjdG9ncmFtIGZvciBhIG11dGFudCBzdHJhaW4uIFlvdSBjYW4gdGVsbCBiZWNhdXNlIHRoZSBwYXR0ZXJuIG9mIGFjdGl2aXR5IHNlZW1zIHRvIGJlIHJhbmRvbSAoYXMgb3Bwb3NlZCB0byBBLCB3aGVyZSB0aGVyZSBhcmUgY2xlYXJseSBkZW1hcmNhdGVkIHBlcmlvZHMgb2YgYWN0aXZpdHkgKGJsYWNrIGJhcnMpIGFuZCBwZXJpb2RzIG9mIHJlc3QgKG5vIGJhcnMpLg==
Cg==UmVhZCBvbiBmb3IgbW9yZSBleGFtcGxlcyBvZiBiZWhhdmlvciBnZW5lcy4=[Qq]
[c]IEVudGVyIHRoZSB3b3Jk[Qq]
[c]ICo=[Qq]
[f]IE5vLiBOb3RpY2UgaG93ICYjODIyMDtBJiM4MjIxOyBoYXMgY29udGlndW91cyBwZXJpb2RzIG9mIGFjdGl2aXR5LCBmb2xsb3dlZCBieSBhY3Rpdml0aWVzIG9mIHF1aWV0LiBUaGlzIGluZGljYXRlcyB0aGF0IHRoZSBjaXJjYWRpYW4gcmh5dGhtIGlzIHdvcmtpbmcsIGFuZCB0aGUgbWljZSBhcmUgaGF2aW5nIHBlcmlvZHMgb2Ygd2FrZWZ1bG5lc3MgKGR1cmluZyB0aGUgbmlnaHQpIGFuZCBzbGVlcCAobW9zdGx5IGR1cmluZyB0aGUgZGF5KS4=
Cg==UmVhZCBvbiBmb3IgbW9yZSBleGFtcGxlcyBvZiBiZWhhdmlvciBnZW5lcy4=[Qq]
[/qwiz]
What’s the takeaway? A mutation in a gene can lead to altered behavior.
4b. Genes Influencing Foraging Behavior in Drosophila larvae
 Here’s another example of a behavior gene in Drosophila.
Here’s another example of a behavior gene in Drosophila.
Drosophila larvae feed on yeast growing on fruit. Some larvae are “rovers,” and travel relatively long distances when foraging for food, while others are “sitters.” These individuals move much less while foraging. Note that these behavioral phenotypes aren’t generalizable to any general level of activity on the part of the larvae. When the larvae aren’t eating, there’s no difference in the amount they move around.
[qwiz qrecord_id=”sciencemusicvideosMeister1961-Drosophila Foraging”]
[h]Drosophila Foraging: Sitters and Rovers
[q labels = “top”]Just to make sure you’re getting this, drag the labels “rover” and “sitter” to the appropriate sites on the graph below.
[l]rover
[fx] No, that’s not correct. Please try again.
[f*] Excellent!
[l]sitter
[fx] No, that’s not correct. Please try again.
[f*] Correct!
[q]Now let’s look at some data from crosses between these strains. Based on these data, which allele is dominant?
[c]U2l0dGVy[Qq]
[f]Tm8uIExvb2sgYXQgY3Jvc3NlcyAzIGFuZCA0LiBOb3csIGxvb2sgYXQgdGhlIG1lYW4gcGF0aCBsZW5ndGhzIGZvciBib3RoIHRoZSBtYWxlcyBhbmQgdGhlIGZlbWFsZXMuIFlvdSBjYW4gc2VlIHRoYXQgdGhlIHBhdGggbGVuZ3RoIGZvciB0aGUgaHlicmlkIHJvdmVyL3NpdHRlcnMgaXMgdGhlIHNhbWUgYXMgdGhlIHBhdGggbGVuZ3RoIGZvciB0aGUgcHVyZWJyZWQgcm92ZXJzIChpbiByb3cgMikuIFRoYXQgaW5kaWNhdGVzIHRoYXQgdGhlIA==cm92ZXI=IHBoZW5vdHlwZSBoYXMgdG8gYmUgZG9taW5hbnQgb3ZlciA=c2l0dGVyLg==[Qq]
[c]Um92 ZXI=[Qq]
[f]RXhjZWxsZW50LiBZb3UgY2FuIHNlZSB0aGF0IHRoZSBwYXRoIGxlbmd0aCBmb3IgdGhlIGh5YnJpZCByb3Zlci9zaXR0ZXJzIChyb3dzIDMgYW5kIDQpIGlzIHRoZSBzYW1lIGFzIHRoZSBwYXRoIGxlbmd0aCBmb3IgdGhlIHB1cmVicmVkIHJvdmVycyAoaW4gcm93IDIpLiBUaGF0IGluZGljYXRlcyB0aGF0IHRoZSA=cm92ZXI=IHBoZW5vdHlwZSBoYXMgdG8gYmUgZG9taW5hbnQgdG8gc2l0dGVyLg==[Qq]
[q]Let’s see what else we can figure out from this data. Drosophila has an XX, XY sex-determination system (analogous to the one in humans and other mammals). The authors of this study assert that the allele is located on an autosome, and not on the X chromosome. Which row below proves their assertion? See if you can use a Punnett square to support your argument (and do that before looking at the answer).
[c]MQ==[Qq]
[f]Tm8uIENyb3NzIDEgaXMgYmV0d2VlbiB0d28gcHVyZWJyZWQgc2l0dGVyIHBhcmVudHMuIEluIGEgY3Jvc3MgbGlrZSB0aGlzLCB0aGUgYWxsZWxlIGNvdWxkIGJlIGF1dG9zb21hbCBvciBYLWxpbmtlZCwgYW5kIHRoZSBvdXRjb21lIHdvdWxkIGJlIHRoZSBzYW1lLg==
Cg==SGVyZSYjODIxNztzIGEgaGludCBhYm91dCBob3cgdG8gdGhpbmsgYWJvdXQgdGhpczogTGV0JiM4MjE3O3MgbWFrZSBSIHRoZSBzeW1ib2wgdGhhdCByZXByZXNlbnRzIHRoZSBkb21pbmFudCByb3ZlciBhbGxlbGUsIGFuZCByIHRoZSBzeW1ib2wgdGhhdCByZXByZXNlbnRzIHRoZSByZWNlc3NpdmUgc2l0dGVyIGFsbGVsZS4gSWYgdGhlIGFsbGVsZSB3ZXJlIG9uIHRoZSBYIGNocm9tb3NvbWUsIHRoZW4gdGhlIG9ubHkgd2F5IHRoYXQgYSBmZW1hbGUgY291bGQgc2hvdyB0aGUgcmVjZXNzaXZlIHBoZW5vdHlwZSB3b3VsZCBiZSB0byBoYXZlIHRoZSBnZW5vdHlwZSA=WA==cg==[Qq]Xr. See if you can take it from there, and identify which cross has results that would be impossible if the allele were on the X chromosome.
[c]Mg==[Qq]
[f]Tm8uIENyb3NzIDIgaXMgYmV0d2VlbiB0d28gcHVyZWJyZWQgcm92ZXIgcGFyZW50cy4gSW4gYSBjcm9zcyBsaWtlIHRoaXMsIHRoZSBhbGxlbGUgY291bGQgYmUgYXV0b3NvbWFsIG9yIFgtbGlua2VkLCBhbmQgdGhlIG91dGNvbWUgd291bGQgYmUgdGhlIHNhbWUu
Cg==SGVyZSYjODIxNztzIGEgaGludCBhYm91dCBob3cgdG8gdGhpbmsgYWJvdXQgdGhpczogTGV0JiM4MjE3O3MgbWFrZSBSIHRoZSBzeW1ib2wgdGhhdCByZXByZXNlbnRzIHRoZSBkb21pbmFudCByb3ZlciBhbGxlbGUsIGFuZCByIHRoZSBzeW1ib2wgdGhhdCByZXByZXNlbnRzIHRoZSByZWNlc3NpdmUgc2l0dGVyIGFsbGVsZS4gSWYgdGhlIGFsbGVsZSB3ZXJlIG9uIHRoZSBYIGNocm9tb3NvbWUsIHRoZW4gdGhlIG9ubHkgd2F5IHRoYXQgYSBmZW1hbGUgY291bGQgc2hvdyB0aGUgcmVjZXNzaXZlIHBoZW5vdHlwZSB3b3VsZCBiZSB0byBoYXZlIHRoZSBnZW5vdHlwZSA=WA==cg==[Qq]Xr. See if you can take it from there, and identify which cross has results that would be impossible if the allele were on the X chromosome.
[c]Mw ==[Qq]
[f]RXhjZWxsZW50LiBXZSBhbHJlYWR5IGtub3cgdGhhdCB0aGUgc2l0dGVyIGFsbGVsZSBpcyByZWNlc3NpdmUuIExldCYjODIxNztzIG1ha2UgUiB0aGUgc3ltYm9sIHRoYXQgcmVwcmVzZW50cyB0aGUgcm92ZXIgYWxsZWxlLCBhbmQgciB0aGUgc3ltYm9sIHRoYXQgcmVwcmVzZW50cyB0aGUgc2l0dGVyIGFsbGVsZS4gSWYgdGhlIGFsbGVsZSB3ZXJlIG9uIHRoZSBYIGNocm9tb3NvbWUsIHRoZW4gdGhlIG9ubHkgd2F5IHRoYXQgYSBmZW1hbGUgY291bGQgc2hvdyB0aGUgcmVjZXNzaXZlIHBoZW5vdHlwZSB3b3VsZCBiZSB0byBoYXZlIHRoZSBnZW5vdHlwZSA=WA==cg==WA==cg==[Qq]. If that were the case, then a cross between a female sitter and a male rover would be represented by this Punnett square.
| Xr | Xr | |
| XR | XRXr | XRXr |
| Y | XrY | XrY |
All of the males would inherit the sitter allele from their mother…which is NOT what we see. Therefore we know that the allele is not on the X chromosome.
[c]NA==[Qq]
[f]Tm8uIFdlIGFscmVhZHkga25vdyB0aGF0IHRoZSBzaXR0ZXIgYWxsZWxlIGlzIHJlY2Vzc2l2ZS4gTGV0JiM4MjE3O3MgbWFrZSBSIHRoZSBzeW1ib2wgdGhhdCByZXByZXNlbnRzIHRoZSByb3ZlciBhbGxlbGUsIGFuZCByIHRoZSBzeW1ib2wgdGhhdCByZXByZXNlbnRzIHRoZSBzaXR0ZXIgYWxsZWxlLiBJZiB0aGUgYWxsZWxlIHdlcmUgb24gdGhlIFggY2hyb21vc29tZSwgdGhlbiB0aGUgZmF0aGVyJiM4MjE3O3MgZ2Vub3R5cGUgd291bGQgaGF2ZSB0byBiZSA=WA==cg==WS7CoA==VGhlIG1vdGhlciwgd2hvIHNob3dzIHRoZSBkb21pbmFudCByb3ZlciBwaGVub3R5cGUsIGhhcyB0byBiZSA=[Qq]XRXR (because the parents are true breeding (not heterozygous). Here’s the cross:
| XR | XR | |
| Xr | XRXr | XRXr |
| Y | XRY | XRY |
All of the offspring would be rovers. But we get the same result if we assume that the allele is autosomal. Then our cross is
| R | R | |
| r | Rr | Rr |
| r | Rr | Rr |
So, cross 4 doesn’t prove anything one way or the other. But one cross would rule out sex-linked inheritance. Which one?
[q]In fact, the inheritance of the rover allele unfolds in a developmentally complex way. While the rover allele acts as a dominant allele in larvae, after development to adult, heterozygotes show an intermediate behavioral phenotype, not moving around as much as homozygous rovers while feeding, but moving around more than homozygous sitters.
On the next card, we can look at how the rover gene works in a population genetics/evolutionary context.
[q labels = “top”]Because behavior has a genetic basis, it can ________. In microevolutionary terms, evolution can be seen as an increase or decrease in ______ frequencies within a gene _____, and that’s exactly what’s been observed with the rover and sitter alleles in experimental populations of Drosophila. For example, keeping flies in high population densities leads to an increase in the frequency of the rover allele, while keeping flies at low population densities leads to an increase in the sitter allele.
Why? In a population with low population density, a larva will be able to find all the food it needs _________. Moving too much while eating would consume ________ while bringing no benefit in return, possibly reducing overall _________. By contrast, high population density would lead to more_____________ for food. In that context, only by moving more would a larva find enough food to successfully _______ and develop (and pass on its _______ to the next generation). (source: Current Biology, 1 January 1998)
[l]allele
[fx] No, that’s not correct. Please try again.
[f*] Good!
[l]competition
[fx] No, that’s not correct. Please try again.
[f*] Correct!
[l]energy
[fx] No. Please try again.
[f*] Good!
[l]evolve
[fx] No, that’s not correct. Please try again.
[f*] Correct!
[l]fitness
[fx] No, that’s not correct. Please try again.
[f*] Good!
[l]genes
[fx] No. Please try again.
[f*] Great!
[l]grow
[fx] No. Please try again.
[f*] Good!
[l]nearby
[fx] No. Please try again.
[f*] Good!
[l]pool
[fx] No. Please try again.
[f*] Good!
[x]
[/qwiz]
4c. More Proof of Behavior Genes in Dogs, Foxes, and Mice
No one familiar with dogs would find it surprising that behaviors can be selected for in mammals —something that would only be possible if behavior were under genetic control. That’s why Collies like to stalk and chase (because they were bred to be shepherds); why Retrievers are great at fetching, but also submissive enough to give what they’re retrieved to their owners; and why Beagles (which were bred to track prey by scent) have an amazing ability to smell (see this for an article about Canine behavioral genetics, and this for a much more general overview).

Dog evolution, however, has occurred over thousands of years. Can such behaviors be selected for on a shorter timescale? The answer to that question has come through a fox domestication program that began in 1959 at the Institute of Cytology and Genetics in Novosibirsk, Russia. This project, initiated by Dmitri Belyaev and continuing through his successors today, systematically selected foxes that were docile around humans. In each generation, only about 10% of the foxes were allowed to breed. Within several generations, aggressive and fear-avoidant behaviors were eliminated from the experimental population. (See “How to Tame a Fox and Build a Dog” in the American Scientist. For some of the controversy around this work, read this article in the NY Times.
In mice, which have a shorter generation time than foxes, behaviors have been selected for an even shorter timescale. In the 1980s, Carol B Lynch selectively bred house mice for nest-building size, measured by the mass of cotton they pulled into their cages for use in their nests. Lynch took mice and divided them into six populations. Two were control populations. Two were selected for high nest weight, and two were selected for low nest weight. The mice were housed under controlled conditions.
[qwiz qrecord_id=”sciencemusicvideosMeister1961-Selection for Nesting Behavior”]
[h]Divergent selection for Nesting Behavior in Mice
[q labels = “top”]In the graph below, label the results of natural selection: high-weight nesters and low-weight nesters. Also, label the control.
[l]control
[fx] No. Please try again.
[f*] Correct!
[l]high nest weight
[fx] No, that’s not correct. Please try again.
[f*] Good!
[l]low nest weight
[fx] No. Please try again.
[f*] Correct!
[q labels = “top”]Lynch also studied variation in nest-building behavior in mice in the wild. She trapped mice from five states in North America: Maine, Connecticut, Virginia, Georgia, and Florida. She paired mice from the same location to create lab-bred populations, raised under identical conditions, and then observed their nest-building behavior under cold and warm conditions.
To keep this from being a test of geography, I’ve provided a map showing the location of these states. Drag the locations and temperatures to the right positions, and then we’ll analyze the results in the next slide.
[l]Connecticut
[fx] No, that’s not correct. Please try again.
[f*] Great!
[l]Florida
[fx] No. Please try again.
[f*] Great!
[l]Georgia
[fx] No. Please try again.
[f*] Correct!
[l]Maine
[fx] No, that’s not correct. Please try again.
[f*] Correct!
[l]Virginia
[fx] No. Please try again.
[f*] Good!
[l]5°C
[fx] No. Please try again.
[f*] Great!
[l]22°C
[fx] No, that’s not correct. Please try again.
[f*] Correct!
[q labels = “top”]Let’s think about the meaning of Lynch’s results. What Lynch had identified was something that population geneticists would call clinal variation (or just a cline). A cline is “a measurable gradient in a single character (or biological trait) of a species across its geographical range” (Wikipedia). We know that the behavior had to have a __________ basis. The evidence for this is that populations from Maine and Florida (to use the extremes), when tested at the same ______________, still showed differences in behavior. At the same time, the ______________ influences the behavior, because all the mice, regardless of geographical origin, built heavier ___________ when it was cold than when it was ___________. Speaking in evolutionary terms, it’s clear that each local population had become _____________to its local environment. In this case, the adaptation was a specific type of ______________.
[l]adapted
[fx] No. Please try again.
[f*] Excellent!
[l]behavior
[fx] No. Please try again.
[f*] Great!
[l]environment
[fx] No. Please try again.
[f*] Good!
[l]genetic
[fx] No. Please try again.
[f*] Good!
[l]nests
[fx] No, that’s not correct. Please try again.
[f*] Great!
[l]temperature
[fx] No. Please try again.
[f*] Excellent!
[l]warm
[fx] No. Please try again.
[f*] Correct!
[/qwiz]
5. Fidelity and Parental Care in Voles
The photo below shows a triple test chamber. It divides the space into three regions, but openings allow a test animal to move from one chamber to the next.
The animal that’s the subject of this experiment is a male vole, a mouse-like member of the rodent family. What’s being tested is fidelity (loyalty to a mate). A mated couple is placed at one end of the chamber. The test vole’s mate is tethered by a chain to one of the chamber walls (imagine a dog with a collar and a leash). Unlike his partner, the male test vole is untethered, and free to move from chamber to chamber. If a new female vole is tethered to the wall of the chamber on the opposite end, what will the test vole do? Will it show fidelity to its partner? Or will he investigate this new arrival?
The answer depends on the species of vole.
Central North America (the Midwest) has two species of voles that behave differently with respect to fidelity and parental care. Prairie voles (Microtus ochrogaster) reside in dry fields covered in grasses and weeds, where they make shallow, underground burrows and runways. Meadow voles (Microtus pennsylvanicus) prefer areas that are moister than those that Prairie voles prefer, and also inhabit woodlands.
Prairie voles are monogamous, and the males are attentive parents. After mating, males participate in rearing the young, hovering over them, licking them, and defending their nests from intruders. Meadow voles, by contrast, are more solitary, with the males playing no role in child-rearing.
[qwiz qrecord_id=”sciencemusicvideosMeister1961-Mating and Parental Care in Voles”]
[h]Mating and Parental Care in Prairie and Meadow Voles
[q]Now that you’ve learned about the differences between Prairie Voles and Meadow voles, take a look at the graph below, which shows data from a partner preference test (the one described above). The Y axis shows time in contact with either a partner (black)) or novel mouse (white). Which species (1 or 2) is the meadow vole?
[c]McKgIMKgIA==[Qq][c]Mg ==
Cg==[Qq][f]Tm8uIFNwZWNpZXMgMSBpcyBhIHByYWlyaWUgdm9sZSwgd2hpY2ggeW91IGNhbiB0ZWxsIGJlY2F1c2UgaXQmIzgyMTc7cyBzcGVuZGluZyBtdWNoIG1vcmUgdGltZSBpbiBjb250YWN0IHdpdGggaXRzIHBhcnRuZXIsIGFuZCBtdWNoIGxlc3MgdGltZSBtZWV0aW5nIHRoZSBub3ZlbCBwYXJ0bmVyIHBsYWNlZCBvbiB0aGUgb3RoZXIgc2lkZSBvZiBpdHMgY2hhbWJlci4gU3BlY2llcyAyIGlzIHRoZSBtdWNoIGxlc3MgbW9ub2dhbW91cyBhbmQgbXVjaCBtb3JlIHNvbGl0YXJ5IG1lYWRvdyB2b2xlLCB3aGljaCB5b3UgY2FuIHRlbGwgYnkgMSkgdGhlIGZhY3QgdGhhdCwgY29tcGFyZWQgdG8gdGhlIHByYWlyaWUgdm9sZSwgaXQgc3BlbmRzIG11Y2ggbGVzcyB0aW1lIGluIGNvbnRhY3Qgd2l0aCBpdHMgbWF0ZSwgYW5kIDIpIGl0JiM4MjE3O3MgcmVsYXRpdmVseSBtdWNoIA==bW9yZQ==IGxpa2VseSB0byBzaG93IGludGVyZXN0IGluIGEgbmV3IHBhcnRuZXIu[Qq]
[f]RXhjZWxsZW50LiBDb21wYXJlZCB0byBzcGVjaWVzIDEsIHNwZWNpZXMgMiBpcyBtdWNoIGxlc3Mgc29jaWFsIChzcGVuZGluZyBtdWNoIGxlc3MgdGltZSB3aXRoIGl0cyBtYXRlLiBJdCYjODIxNztzIGFsc28gc2hvd2luZyByZWxhdGl2ZWx5IGxlc3MgaW50ZXJlc3QgaW4gaXRzIHBhcnRuZXIsIGFuZCBtb3JlIGludGVyZXN0IGluIHRoZSBuZXdseSBpbnRyb2R1Y2VkIHZvbGUgb24gdGhlIG90aGVyIHNpZGUgb2YgdGhlIGNoYW1iZXIuIFRoYXQmIzgyMTc7cyBleGFjdGx5IHdoYXQgeW91JiM4MjE3O2QgZXhwZWN0IG9mIHRoZSBzb2xpdGFyeSBhbmQgbm9ubW9ub2dhbW91cyBtZWFkb3cgdm9sZS4=[Qq]
[q]Prairie voles and meadow voles are both in the genus Microtus, which means that they’re closely related. The difference in their behavior is related to a hormone that’s important in osmoregulation: ADH, or anti-diuretic hormone. This hormone is also called vasopressin, and it’s released during mating, where it binds to V1aR, the vasopressin receptor. The more vasopressin binds to V1aR, the more post-mating social behavior seems to be expressed.
Both vole species produce vasopressin. Where they differ is in the amount of V1aR that gets expressed in the cells of their brain. In the diagram at left, darker regions indicate a high density of V1aR in two brain regions (indicated by their initials, LS and VP). Which species is the meadow vole?
[c]QQ==[Qq]
[f]Tm8uIFRoZSBtZWFkb3cgdm9sZSBpcyBzb2xpdGFyeS4gSXQgd291bGQgYmUgbGVzcyBzZW5zaXRpdmUgdG8gdmFzb3ByZXNzaW4sIGFuZCB0aGF0IHNlbnNpdGl2aXR5IHdvdWxkIHJlc3VsdCBmcm9tIGhhdmluZyBmZXdlciByZWNlcHRvcnMgKGFzIHNob3duIGluICYjODIyMDtCJiM4MjIxOykuIFRoZSBicmFpbiB3aXRoIGxvdHMgb2YgcmVjZXB0b3JzIChicmFpbiBBKSBiZWxvbmdzIHRvIGEgcHJhaXJpZSB2b2xlLg==[Qq]
[c]Qg ==[Qq]
[f]RXhjZWxsZW50LiBUaGUgbWVhZG93IHZvbGUsIHdpdGggaXRzIHNvbGl0YXJ5IGJlaGF2aW9yLCBzZWVtcyBsaWtlbHkgdG8gaGF2ZSBmZXdlciB2YXNvcHJlc3NpbiByZWNlcHRvcnMgdGhhbiB0aGUgcHJhaXJpZSB2b2xlLg==[Qq]
[q]If you give male prairie voles a drug that inhibits the receptor for vasopressin, it’s most likely that
[c]dGhleSB3aWxsIGJlY29tZSBtb3JlIG1vbm9nYW1vdXMu[Qq]
[f]Tm8uIFRoZSBtYWxlIHBhaXIgYm9uZGluZyB0aGF0JiM4MjE3O3Mgc2VlbiBpbiBwcmFpcmllIHZvbGVzIHNlZW1zIHRvIGJlIGFzc29jaWF0ZWQgd2l0aCBpdHMgYWJ1bmRhbnQgZXhwcmVzc2lvbiBvZiBWMWFSICh0aGUgdmFzb3ByZXNzaW4gcmVjZXB0b3IpLiBJZiB5b3UgYmxvY2sgdGhhdCByZWNlcHRvciwgdGhlbiB3aGF0ZXZlciBicmFpbiBjaXJjdWl0cyB1bmRlcmx5IG1vbm9nYW15IHdvbiYjODIxNzt0IGJlIGFjdGl2YXRlZC4gSWYgdGhhdCYjODIxNztzIHRoZSBjYXNlLCB5b3UmIzgyMTc7ZCBleHBlY3QgdGhlIHByYWlyaWUgdm9sZXMgd2l0aCBibG9ja2VkIHZhc29wcmVzc2luIHJlY2VwdG9ycyB0byBiZWNvbWUgbGVzcw==IG1vbm9nYW1vdXMu[Qq]
[c]dGhleSBmYWlsIHRvIGZvcm0gcGFp ciBib25kcyBhZnRlciBtYXRpbmcu
[f]RXhjZWxsZW50ISBUaGUgbWFsZSBwYWlyIGJvbmRpbmcgdGhhdCYjODIxNztzIHNlZW4gaW4gcHJhaXJpZSB2b2xlcyBzZWVtcyB0byBiZSBhc3NvY2lhdGVkIHdpdGggaXRzIGFidW5kYW50IGV4cHJlc3Npb24gb2YgVjFhUiAodGhlIHZhc29wcmVzc2luIHJlY2VwdG9yKS4gSWYgeW91IGJsb2NrIHRoYXQgcmVjZXB0b3IsIHRoZW4gd2hhdGV2ZXIgYnJhaW4gY2lyY3VpdHMgdW5kZXJseSBtb25vZ2FteSB3b24mIzgyMTc7dCBiZSBhY3RpdmF0ZWQuIElmIHRoYXQmIzgyMTc7cyB0aGUgY2FzZSwgeW91JiM4MjE3O2QgZXhwZWN0IHRoZSBwcmFpcmllIHZvbGVzIHdpdGggYmxvY2tlZCB2YXNvcHJlc3NpbiByZWNlcHRvcnMgdG8gYmVjb21lIA==bGVzcw==IG1vbm9nYW1vdXMu[Qq]
[q]To test the idea that higher vasopressin receptor levels increase post-mating bonding behaviors in males, researchers inserted the gene for increased V1aR (the vasopressin receptor) into meadow voles. Based on what you’ve read, meadow voles with a gene that increased the expression of the vasopressin receptor should
[c]YmUgbW9yZSBt b25vZ2Ftb3Vz[Qq]
[f]VGhhdCYjODIxNztzIGNvcnJlY3QuIFdpdGggbW9yZSBWMWFSLCB0aGUgbWVhZG93IHZvbGVzIHdpbGwgc3RhcnQgdG8gYmVoYXZlIGxpa2UgdGhlaXIgbW9yZSBtb25vZ2Ftb3VzIHJlbGF0aXZlcywgcHJhaXJpZSB2b2xlcyAod2hvIGFscmVhZHkgZXhwcmVzcyBoaWdoIGxldmVscyBvZiB0aGUgVjFhUiBnZW5lKS4=[Qq]
[c]YmUgbGVzcyBtb25vZ2Ftb3Vz[Qq]
[f]Tm8uIFJlbWVtYmVyIHRoYXQgdGhlIGRpZmZlcmVuY2UgYmV0d2VlbiBwcmFpcmllIHZvbGVzIGFuZCBtZWFkb3cgdm9sZXMgaXMgdGhhdCB0aGUgcHJhaXJpZSB2b2xlcyBoYXZlIGEgaGlnaGVyIGRlbnNpdHkgb2YgVjFhUi4gSWYgZ2VuZXRpYyBtb2RpZmljYXRpb24gbWFrZXMgYSBtZWFkb3cgdm9sZSBtb3JlIGxpa2UgYSBwcmFpcmllIHZvbGUsIHRoZW4geW91IHdvdWxkIGV4cGVjdCB0aGF0IHRoZSBtb2RpZmllZCBtZWFkb3cgdm9sZSB3b3VsZCBiZWhhdmUgbGlrZSBhIHByYWlyaWUgdm9sZSwgYW5kIGJlIG1vcmUgbW9ub2dhbW91cy4=[Qq]
[/qwiz]
To learn more about vasopressin and monogamy in voles, read this article in Scientific American.
6. The Genetic Basis of Behavior: Checking Understanding
[qwiz qrecord_id=”sciencemusicvideosMeister1961-Genetic Basis of Behavior”]
[h]The Genetic Basis of Behavior
[i]Biohaiku
Our Brain’s Receptors
Bonding with Vasopressin
Is that what love is?
[q] The various case studies we’ve seen above, involving animals as diverse as Drosophila, dogs, foxes, mice, and voles, establish that behavior can have a [hangman] basis and that it can [hangman] over time.
[c]IGdlbmV0aWM=[Qq]
[f]IENvcnJlY3Qh[Qq]
[c]IGV2b2x2ZQ==[Qq]
[f]IEdyZWF0IQ==[Qq]
[q] In Drosophila, the per gene influences [hangman] rhythms.
[c]IGNpcmNhZGlhbg==[Qq]
[f]IEdvb2Qh[Qq]
[q] The graph below showed how genes could control [hangman] behavior in Drosophila larvae. Some larvae (such as those in group 1) are [hangman], and move relatively little when looking for food. Others (such as those in group 2) are [hangman], and travel much further.
[c]IGZvcmFnaW5n[Qq]
[f]IENvcnJlY3Qh[Qq]
[c]IHNpdHRlcnM=[Qq]
[f]IEV4Y2VsbGVudCE=[Qq]
[c]IHJvdmVycw==[Qq]
[f]IEdyZWF0IQ==[Qq]
[q] Based on data from experimental crosses, it turns out that the Rover allele is [hangman] and [hangman](not on the X [hangman].
[c]IGRvbWluYW50[Qq]
[f]IENvcnJlY3Qh[Qq]
[c]IGF1dG9zb21hbA==[Qq]
[f]IEV4Y2VsbGVudCE=[Qq]
[c]IGNocm9tb3NvbWU=[Qq]
[f]IEdvb2Qh[Qq]
[q] Researchers have also demonstrated that the frequency of the rover and sitter alleles could [hangman] over time in response to the environment. For example, in high population densities, the allele for rover [hangman] in frequency, because those larvae who move further in search of food will [hangman] others who move less.
[c]IGV2b2x2ZQ==[Qq]
[f]IENvcnJlY3Qh[Qq]
[c]IGluY3JlYXNlcw==[Qq]
[f]IEdyZWF0IQ==[Qq]
[c]b3V0Y29tcGV0ZQ==[Qq]
[q] The existence of clearly defined behavioral traits in dogs is additional evidence that [hangman] can determine (or, at least, influence) behavior. This was further confirmed by Dimitri Belyaev’s experimental breeding program with foxes. Over several generations, Belyaev and his collaborators were able to eliminate [hangman] behaviors and produce a population of foxes that behaved in a very dog-like manner.
[c]IGdlbmVz[Qq]
[f]IENvcnJlY3Qh[Qq]
[c]IGFnZ3Jlc3NpdmU=[Qq]
[f]IEV4Y2VsbGVudCE=[Qq]
[q] In breeding experiments with mice, Carol B Lynch was able to select for divergent types of [hangman] building behaviors.
[c]IG5lc3Q=[Qq]
[f]IEV4Y2VsbGVudCE=[Qq]
[q]Lynch was also able to demonstrate clinal [hangman] in nest-building behavior in different populations of mice distributed along the East coast of North America. The key thing to note is the [hangman] basis for these behaviors, which persisted even when mice from different regions built their nests in similar conditions, above all similar [hangman].
[c]dmFyaWF0aW9u[Qq]
[c]Z2VuZXRpYw==[Qq]
[c]dGVtcGVyYXR1cmU=[Qq]
[q] Work with prairie voles and meadow voles has also established the genetic and physiological basis for partner [hangman] and [hangman] care (at least in voles). Prairie voles are much more monogamous than meadow voles, and the difference lies in the density of [hangman] for the hormone vasopressin that gets expressed in the [hangman] of these rodents. Solitary meadow voles that have been genetically engineered to express [hangman] of the vasopressin receptor start acting more like prairie voles. Conversely, prairie voles that are given drugs that block the vasopressin receptor become [hangman] monogamous.
[c]IGZpZGVsaXR5[Qq]
[f]IEdyZWF0IQ==[Qq]
[c]IHBhcmVudGFs[Qq]
[f]IEdvb2Qh[Qq]
[c]IHJlY2VwdG9ycw==[Qq]
[f]IEdvb2Qh[Qq]
[c]IGJyYWlucw==[Qq]
[f]IENvcnJlY3Qh[Qq]
[c]IG1vcmU=[Qq]
[f]IENvcnJlY3Qh[Qq]
[c]IGxlc3M=[Qq]
[f]IENvcnJlY3Qh[Qq]
[q] The diagram below shows the results of a mate choice experiment in two species of voles. Which species is more monogamous?
[textentry single_char=”true”]
[c]ID E=[Qq]
[f]IENvcnJlY3QuIFNwZWNpZXMgMSBpcyBzcGVuZGluZyBtb3JlIHRpbWUgd2l0aCBpdHMgbWF0ZSBhbmQgaXMgcmVsYXRpdmVseSBsZXNzIGludGVyZXN0ZWQgaW4gdGhlIG5vdmVsIG1vdXNlLiBCb3RoIGFyZSBpbmRpY2F0b3JzIG9mIG1vbm9nYW15Lg==[Qq]
[c]IEVudGVyIHRoZSB3b3Jk[Qq]
[c]ICo=[Qq]
[f]IE5vLiBUaGUga2V5IHRoaW5nIHRvIGxvb2sgZm9yIGlzIHdoaWNoIHNwZWNpZXMgaXMgc3BlbmRpbmcgbW9yZSB0aW1lIHdpdGggaXRzIHBhcnRuZXIsIGFuZCBzaG93aW5nIHJlbGF0aXZlbHkgbGVzcyBpbnRlcmVzdCBpbiB0aGUgbm92ZWwgbW91c2Uu[Qq]
[q] The diagram below shows the results of a mate choice experiment in two species of voles. If you examined the brains of each species, which would have fewer vasopressin receptors?
[textentry single_char=”true”]
[c]ID I=[Qq]
[f]IEV4Y2VsbGVudC4gU3BlY2llcyAyIGlzIG1vcmUgc29saXRhcnkgKHNwZW5kaW5nIGxlc3MgdGltZSB3aXRoIGl0cyBtYXRlKSBhbmQgbGVzcyBtb25vZ2Ftb3VzIChzaG93aW5nIHJlbGF0aXZlbHkgbW9yZSBpbnRlcmVzdCBpbiB0aGUgbm92ZWwgbW91c2UpLiBJbiB2b2xlcywgdGhlIHZhc29wcmVzc2luIGhvcm1vbmUgaXMgY29ubmVjdGVkIHdpdGggYm9uZGluZyBiZWhhdmlvciwgYW5kLCBiYXNlZCBvbiBzcGVjaWVzIDImIzgyMTc7cyBiZWhhdmlvciwgaXQgc2VlbXMgbGlrZWx5IHRvIGhhdmUgZmV3ZXIgcmVjZXB0b3JzIGZvciBhIGhvcm1vbmUgdGhhdCB3b3VsZCBsZWFkIHRvIGJvbmRpbmcu[Qq]
[c]IEVudGVyIHRoZSB3b3Jk[Qq]
[c]ICo=[Qq]
[f]IE5vLiBTcGVjaWVzIDEgbG9va3MgdG8gYmUgaGlnaGx5IHNvY2lhbCBhbmQgbW9ub2dhbW91cy4gU2luY2UgaW4gdm9sZXMsIHRoZXNlIGJlaGF2aW9ycyBhcmUgYXNzb2NpYXRlZCB3aXRoIHRoZSBob3Jtb25lIHZhc29wcmVzc2luLCBpdCBzZWVtcyBsaWtlbHkgdGhhdCB0aGlzIGhvcm1vbmUgaXMgYmluZGluZyB3aXRoIG5lcnZlIGNlbGxzIHRoYXQgYXJlIGVsaWNpdGluZyB0aGVzZSBiZWhhdmlvcnMuIElmIHRoYXQmIzgyMTc7cyB0aGUgY2FzZSwgd2hpY2ggc3BlY2llcyB3b3VsZCBoYXZlIGZld2VyIHJlY2VwdG9ycz8=[Qq]
[/qwiz]
What’s next?
- Learning and Orienting (the next tutorial in this module)
- Animal Behavior Menu

