Click here for an overview video of oxidative phosphorylation (covered below)
1. Introduction
In the previous tutorials in this series about cellular respiration, we’ve seen how glycolysis, the link reaction, and the Krebs cycle oxidize food so that the mobile electron carriers NAD+ and FAD can be reduced to NADH and FADH2, respectively.
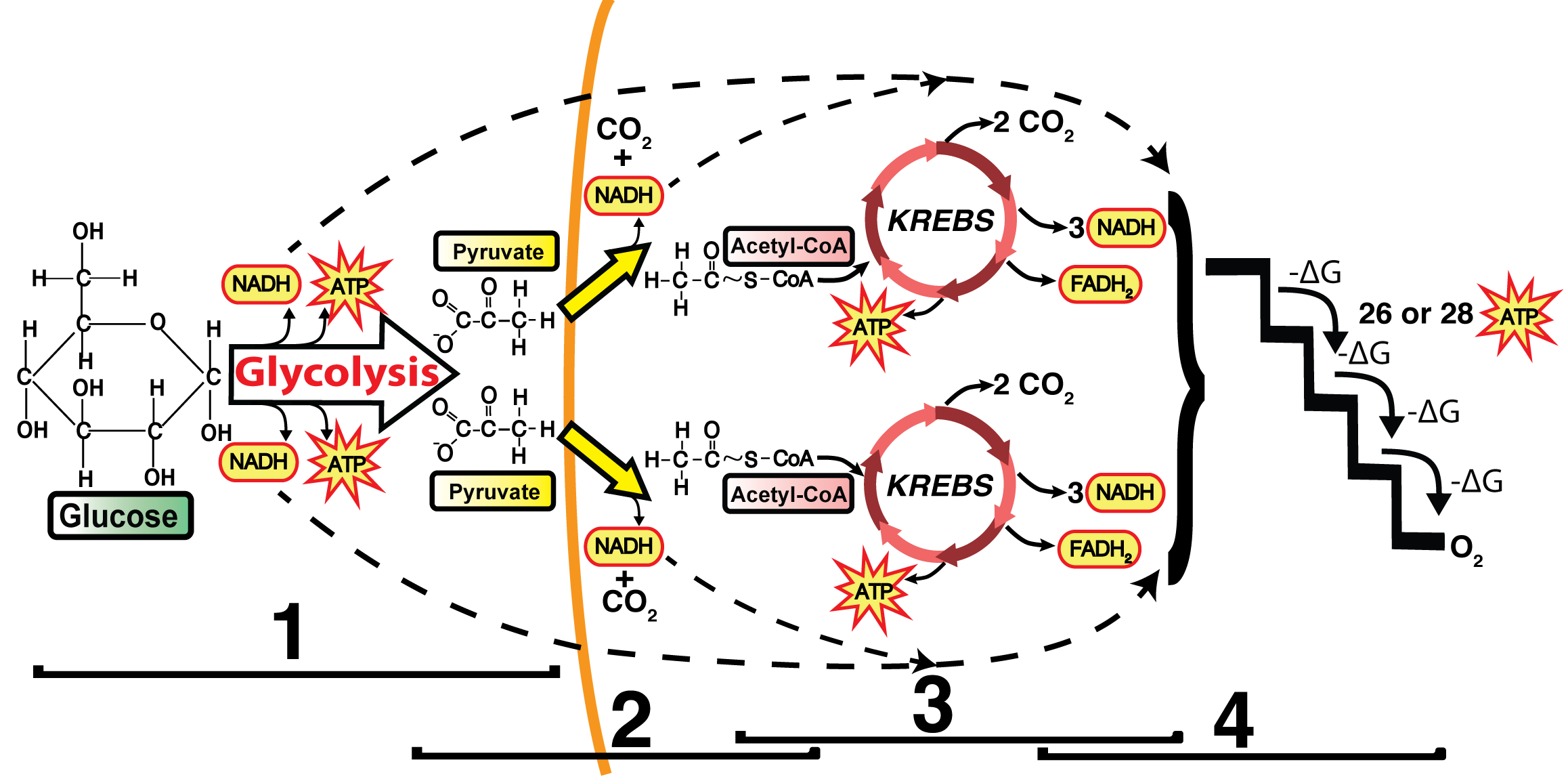
NADH and FADH2 can be thought of as rechargeable batteries that are fully charged. If you took a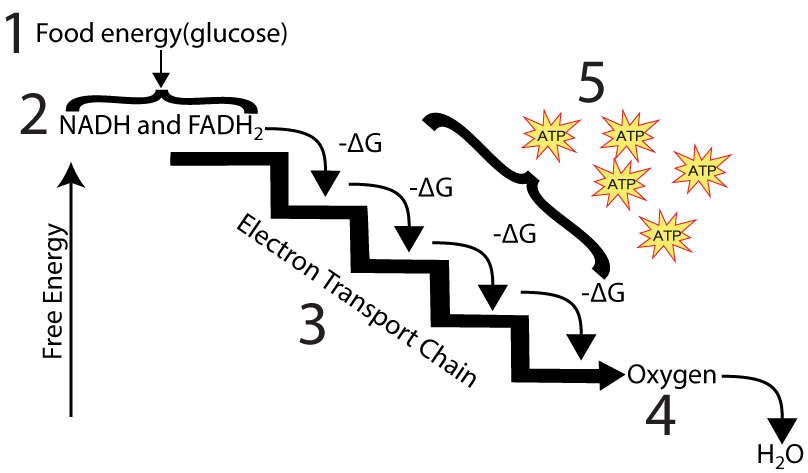 charged-up battery and placed it in a flashlight or a radio, then that battery could release a flow of electrical energy that could, in turn, power some work: providing light in the case of the flashlight or converting radio waves into sound in the case of the radio. In cells, an analogous thing happens. As shown in a schematic form on the left, NADH and FADH2 deliver electrons to the electron transport chain, a series of proteins that are embedded in the inner membrane of the mitochondria.
charged-up battery and placed it in a flashlight or a radio, then that battery could release a flow of electrical energy that could, in turn, power some work: providing light in the case of the flashlight or converting radio waves into sound in the case of the radio. In cells, an analogous thing happens. As shown in a schematic form on the left, NADH and FADH2 deliver electrons to the electron transport chain, a series of proteins that are embedded in the inner membrane of the mitochondria.
As electrons flow along the chain, the proteins in the chain do the work of setting up the conditions for ATP creation. Let’s see how this process works.
2. Mitochondria: Structure and Function
ATP synthesis in the mitochondria is a beautiful example of one of biology’s most important ideas: the relationship between structure and function. The quiz below will let you master the key structures involved. Then we’ll move on to the process.
[qwiz qrecord_id=”sciencemusicvideosMeister1961-Mitochondrial Structure for ETC (M10)”]
[h]Interactive Diagrams: Mitochondrial Structure
[i]
[q labels = “top”]
[l]cell exterior
[f*] Excellent!
[fx] No, that’s not correct. Please try again.
[l]cell membrane
[f*] Excellent!
[fx] No. Please try again.
[l]cytoplasm
[f*] Excellent!
[fx] No, that’s not correct. Please try again.
[l]inner membrane
[f*] Good!
[fx] No. Please try again.
[l]intermembrane space
[f*] Good!
[fx] No, that’s not correct. Please try again.
[l]matrix
[f*] Excellent!
[fx] No, that’s not correct. Please try again.
[l]mitochondrion
[f*] Correct!
[fx] No. Please try again.
[l]outer membrane
[f*] Correct!
[fx] No. Please try again.
[q labels = “top”]
Here’s a more realistic depiction of a mitochondrion. You should be able to label all of the parts, with the possible exception of one. ATP Synthase is a protein channel and an enzyme that’s embedded in the mitochondrial membrane. See if you can figure it out as you label the diagram below.
[l]ATP Synthase
[f*] Excellent!
[fx] No, that’s not correct. Please try again.
[l]chromosome
[f*] Great!
[fx] No. Please try again.
[l]cytoplasm
[f*] Good!
[fx] No, that’s not correct. Please try again.
[l]inner membrane
[f*] Great!
[fx] No, that’s not correct. Please try again.
[l]intermembrane space
[f*] Excellent!
[fx] No. Please try again.
[l]matrix
[f*] Good!
[fx] No. Please try again.
[l]outer membrane
[f*] Great!
[fx] No, that’s not correct. Please try again.
[q] Now we’re going to zoom in on the outer boundary of the mitochondrion. Just focus on the letters (you’ll learn about all the numbered parts later). Which letter is the inner membrane?
[textentry single_char=”true”]
[c]IE Q=
[f]IEV4Y2VsbGVudC4gJiM4MjIwO0QmIzgyMjE7IGlzIHRoZSBpbm5lciBtaXRvY2hvbmRyaWFsIG1lbWJyYW5lLg==[Qq]
[c]IEVudGVyIHdvcmQ=[Qq]
[c]ICo=[Qq]
[f]IE5vLiBIZXJlJiM4MjE3O3MgYSBoaW50OiBub3RpY2UgdGhhdCB0aGVyZSBhcmUgdHdvIGxpcGlkIGJpbGF5ZXJzLiBJZiAmIzgyMjA7QSYjODIyMTsgaXMgdGhlIGN5dG9wbGFzbSwgd2hpY2ggbWVtYnJhbmUgaGFzIHRvIGJlIHRoZSBpbm5lciBtZW1icmFuZT8=[Qq]
[q] Which letter is the outer membrane?
[textentry single_char=”true”]
[c]IE I=
[f]IEV4Y2VsbGVudC4gJiM4MjIwO0ImIzgyMjE7IGlzIHRoZSBvdXRlciBtaXRvY2hvbmRyaWFsIG1lbWJyYW5lLg==[Qq]
[c]IEVudGVyIHdvcmQ=[Qq]
[c]ICo=[Qq]
[f]IE5vLiBIZXJlJiM4MjE3O3MgYSBoaW50OiBub3RpY2UgdGhhdCB0aGVyZSBhcmUgdHdvIGxpcGlkIGJpbGF5ZXJzLiBJZiAmIzgyMjA7QSYjODIyMTsgaXMgdGhlIGN5dG9wbGFzbSwgd2hpY2ggbWVtYnJhbmUgaGFzIHRvIGJlIHRoZSBvdXRlciBtZW1icmFuZT8=[Qq]
[q] Which letter indicates the inter-membrane space?
[textentry single_char=”true”]
[c]IE M=
[f]IEV4Y2VsbGVudC4gJiM4MjIwO0MmIzgyMjE7IGlzIHRoZSBpbnRlci1tZW1icmFuZSBzcGFjZS4=[Qq]
[c]IEVudGVyIHdvcmQ=[Qq]
[c]ICo=[Qq]
[f]IE5vLiBIZXJlJiM4MjE3O3MgYSBoaW50OiBub3RpY2UgdGhhdCB0aGVyZSBhcmUgdHdvIGxpcGlkIGJpbGF5ZXJzLiBXaGljaCByZWdpb24gbXVzdCBiZSB0aGUgc3BhY2UgYmV0d2Vlbg==IHRoZXNlIHR3byBtZW1icmFuZXMsIGFsc28ga25vd24gYXMgdGhlICYjODIyMDtpbnRlci1tZW1icmFuZSBzcGFjZS4mIzgyMjE7[Qq]
[q] Which letter indicates the cytoplasm?
[textentry single_char=”true”]
[c]IE E=
[f]IEV4Y2VsbGVudC4gJiM4MjIwO0EmIzgyMjE7IGlzIHRoZSBjeXRvcGxhc20u[Qq]
[c]IEVudGVyIHdvcmQ=[Qq]
[c]ICo=[Qq]
[f]IE5vLiBIZXJlJiM4MjE3O3MgYSBoaW50OiBub3RpY2UgdGhhdCB0aGVyZSBhcmUgdHdvIGxpcGlkIGJpbGF5ZXJzLiBXaGljaCByZWdpb24gaXMgb3V0c2lkZSBvZiB0aGUgb3V0ZXIgbWVtYnJhbmUgKGFuZCBtdXN0LCB0aGVyZWZvcmUsIGJlIHRoZSBjeXRvcGxhc20pPw==[Qq]
[q] Which letter indicates the mitochondrial matrix?
[textentry single_char=”true”]
[c]IE U=
[f]IEV4Y2VsbGVudC4gJiM4MjIwO0UmIzgyMjE7IGlzIHRoZSBtaXRvY2hvbmRyaWFsIG1hdHJpeC4=[Qq]
[c]IEVudGVyIHdvcmQ=[Qq]
[c]ICo=[Qq]
[f]IE5vLiBIZXJlJiM4MjE3O3MgYSBoaW50OiBub3RpY2UgdGhhdCB0aGVyZSBhcmUgdHdvIGxpcGlkIGJpbGF5ZXJzLiBXaGljaCByZWdpb24gaXMgaW5zaWRlIG9mIHRoZSBpbm5lciBtZW1icmFuZSAoYW5kIG11c3QsIHRoZXJlZm9yZSwgYmUgdGhlIG1hdHJpeCwgd2hpY2ggaXMgdGhlIGN5dG9wbGFzbSBvZiB0aGUgbWl0b2Nob25kcmlvbik/[Qq]
[/qwiz]
3. It starts with pumping protons
So, that’s the overall structure. Let’s focus on the electron transport chain, in the inner mitochondrial membrane.
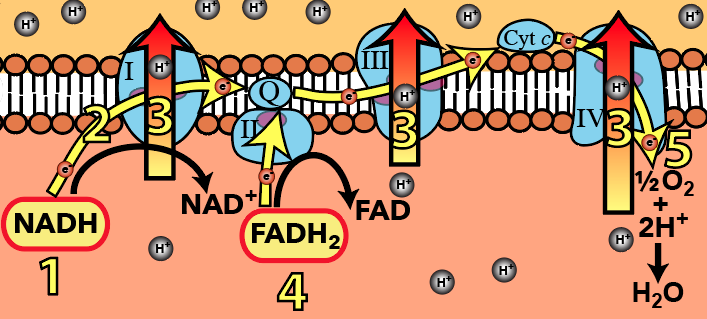
The chain is a series of protein complexes organized in a sequence in the inner mitochondrial membrane. Organized in this way, you can imagine them as a type of molecular “wire” that allows electrons donated by NADH and FADH2 to flow. The electron flow pathway is shown above at number “2,” with “1” being electron donation by NADH, and “4” being electron donation by FADH2. Each of these donations is an oxidation and results in NADH becoming its oxidized counterpart NAD+, and FADH2 becoming FAD. At the very end of the chain (at “5”), electrons flow to oxygen. As oxygen accepts electrons, it also grabs protons from the matrix. This reduces oxygen to water, the second waste product of cellular respiration.
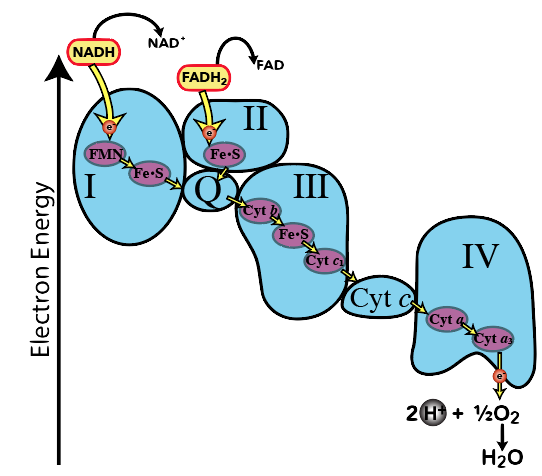 An important feature of the electron transport chain is that the electron carriers are organized in terms of electronegativity. Electronegativity is “the tendency to acquire electrons.” As you move along the electron transport chain, each electron carrier has a greater electronegativity than the one before it. You can see this in the diagram to the left. Inside Complex I you can see the electron carriers FMN and FE.S, which are positioned next to “Q.” “Q” can pull electrons from FE.S (because “Q” has greater electronegativity).FE.S can, in turn, pull electrons from FMN. You can imagine electrons falling “down” an energy gradient. As they fall, they release energy that can be harvested to do work.
An important feature of the electron transport chain is that the electron carriers are organized in terms of electronegativity. Electronegativity is “the tendency to acquire electrons.” As you move along the electron transport chain, each electron carrier has a greater electronegativity than the one before it. You can see this in the diagram to the left. Inside Complex I you can see the electron carriers FMN and FE.S, which are positioned next to “Q.” “Q” can pull electrons from FE.S (because “Q” has greater electronegativity).FE.S can, in turn, pull electrons from FMN. You can imagine electrons falling “down” an energy gradient. As they fall, they release energy that can be harvested to do work.
Looking at this diagram, we can see why oxygen is so important in this last phase of cellular respiration. Oxygen is the final electron acceptor in the electron transport chain. Its electronegativity pulls electrons down the chain. Without oxygen, electrons don’t flow. And if electrons don’t flow, the work that makes ATP synthesis possible won’t happen. Life, very quickly, grinds to a halt.
And what is this work? The work is pumping protons from the mitochondrial matrix (shown here below the inner  membrane) to the intermembrane space (shown above the inner membrane). In this diagram, proton pumps are indicated by the number “3”, and there are three different ones shown: one in Complex I, one in Complex III, and one in Complex IV.
membrane) to the intermembrane space (shown above the inner membrane). In this diagram, proton pumps are indicated by the number “3”, and there are three different ones shown: one in Complex I, one in Complex III, and one in Complex IV.
All of these pumps are doing the same thing, and they’re all powered by the same energy source: the flow of electrons along the electron pathway (again, at number “2”). Note that this is active transport: the pumps are pumping up a concentration gradient, from where protons are less concentrated (in the matrix) to where they’re more concentrated (in the intermembrane space).
4. It ends with making ATP
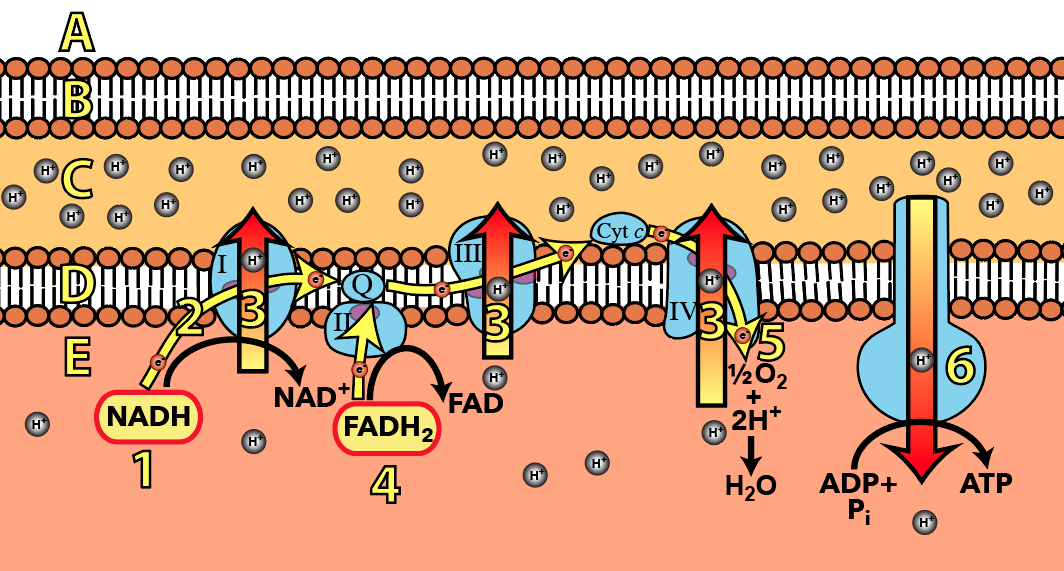 Pumping protons from the matrix (E) to the intermembrane space (C) creates an enormous potential energy gradient that the cell harvests to make ATP. This gradient is itself a product of the structure of the mitochondria. Though protons are small, they’re charged, and the phospholipid bilayer is impermeable to charged particles. Consequently, once protons are pumped into the intermembrane space, they’re effectively trapped there.
Pumping protons from the matrix (E) to the intermembrane space (C) creates an enormous potential energy gradient that the cell harvests to make ATP. This gradient is itself a product of the structure of the mitochondria. Though protons are small, they’re charged, and the phospholipid bilayer is impermeable to charged particles. Consequently, once protons are pumped into the intermembrane space, they’re effectively trapped there.
The gradient has several features. First, it’s a diffusion gradient, caused by two forces. Based on what you’ve read above, you know that protons have been pumped into the intermembrane space. At the same time, when oxygen becomes reduced to water at step “5,” protons are being removed from the matrix. That creates a second force: there’s a voltage gradient across the inner membrane. The positively charged protons are, in a physical sense, motivated to get out of the positively charged intermembrane space (because like charges repel) and into the more negatively charged matrix (because opposite charges attract).
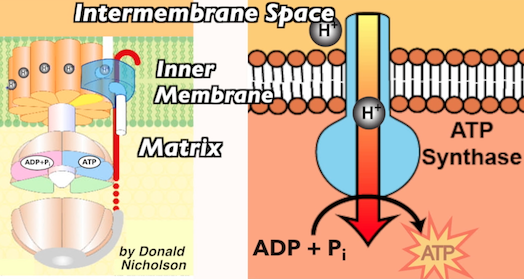 There’s only one way for protons to get back to the matrix, and that’s through a protein channel and an enzyme called ATP synthase. ATP synthase has binding sites for ADP and Pi (inorganic phosphate). When protons diffuse through ATP synthase, their kinetic energy (energy of motion) causes these binding sites to change shape. This catalyzes the formation of a bond between ADP and Pi, transforming them into ATP.
There’s only one way for protons to get back to the matrix, and that’s through a protein channel and an enzyme called ATP synthase. ATP synthase has binding sites for ADP and Pi (inorganic phosphate). When protons diffuse through ATP synthase, their kinetic energy (energy of motion) causes these binding sites to change shape. This catalyzes the formation of a bond between ADP and Pi, transforming them into ATP.
Here’s how I describe this process in my Electron Transport Chain music video:
ATP synthase is embedded in the inner membrane
How it works is so cool it’s insane.
It’s got channels for diffusing protons running right through it.
When cells make ATP, well watch how they do itThe matrix side of ATP synthase has binding sites
For ADP and P which come in and bind.
And as ATP synthase lets protons barge through
Their kinetic energy gets put to use.Like water through a turbine proton movement generates rotation.
Changing synthase’s binding site conformation.
Which catalyzes chemical bond formation.
ADP and P make ATP that energy sensation!
There are a few important biology-vocabulary terms that are connected with this process that you have to know.
- Chemiosmosis: the generation of ATP by the movement of protons from the intermembrane space across the inner mitochondrial membrane (through ATP synthase) during cellular respiration (adapted from Wikipedia).
- Oxidative phosphorylation is the process in which ATP is formed as a result of the transfer of electrons from NADH or FADH2 to O2 by a series of electron carriers (adapted from Biochemistry, 5th edition)
- Proton-motive force is the potential energy stored as a combination of proton and voltage (electrical potential) gradients across the inner membrane of the mitochondrion (see Wikipedia reference above)
5. Quiz. The Electron Transport Chain
Got it? Start with the following quiz.
[qwiz random = “true” qrecord_id=”sciencemusicvideosMeister1961-Electron Transport Chain Quiz (M10)”]
[h]Quiz: The electron transport chain
[i]
[q]In the diagram below, which number represents the part where you’d find ATP synthase?
[textentry single_char=”true”]
[c]Ng ==[Qq]
[f]IFllcywg4oCcNuKAnSBpcyB0aGUgaW5uZXIgbWl0b2Nob25kcmlhbCBtZW1icmFuZSwgd2hpY2ggaXMgd2hlcmUgQVRQIHN5bnRoYXNlIGlzIGxvY2F0ZWQu[Qq]
[c]Kg==[Qq]
[f]Tm8uIEhlcmUmIzgyMTc7cyBhIGhpbnQuIEFUUCBzeW50aGFzZSBnZW5lcmF0ZXMgQVRQIGFzIHByb3RvbnMgZmxvdyBmcm9tIHRoZSBpbnRlcm1lbWJyYW5lIHNwYWNlIHRvIHRoZSBtYXRyaXguIFdoZXJlIHdvdWxkIEFUUCBzeW50aGFzZSBoYXZlIHRvIGJlIGxvY2F0ZWQgdG8gYWxsb3cgZm9yIHRoaXM/
Cg==
[q]In the diagram below, which number represents the part where you’d find the electron transport chain?
[textentry single_char=”true”]
[c]Ng ==[Qq]
[f]IFllcywg4oCcNuKAnSBpcyB0aGUgaW5uZXIgbWl0b2Nob25kcmlhbCBtZW1icmFuZSwgd2hpY2ggaXMgd2hlcmUgdGhlIGVsZWN0cm9uIHRyYW5zcG9ydCBjaGFpbiBpcyBsb2NhdGVkLg==[Qq]
[c]Kg==[Qq]
[f]Tm8uIEhlcmUmIzgyMTc7cyBhIGhpbnQuIFRoZSBlbGVjdHJvbiB0cmFuc3BvcnQgY2hhaW4gcHVtcHMgcHJvdG9ucyBmcm9tIHRoZSBtYXRyaXggdG8gdGhlIGludGVybWVtYnJhbmUgc3BhY2UuIFdoZXJlIHdvdWxkIGl0IGhhdmUgdG8gYmUgbG9jYXRlZCB0byBhbGxvdyBmb3IgdGhpcz8=
Cg==
[q]In the diagram below, which number represents the area that protons get pumped into?
[textentry single_char=”true”]
[c]Nw ==[Qq]
[f]IFllcy4g4oCcN+KAnSBpcyB0aGUgaW50ZXJtZW1icmFuZSBzcGFjZSwgd2hpY2ggaXMgd2hlcmUgcHJvdG9ucyBnZXQgcHVtcGVkIHRvIGNyZWF0ZSBhIGNvbmNlbnRyYXRpb24gZ3JhZGllbnQgZm9yIHN5bnRoZXNpemluZyBBVFAu[Qq]
[c]Kg==[Qq]
[f]Tm8uIEhlcmUmIzgyMTc7cyBhIGhpbnQuIFRoZSBwcm90b25zIHN0YXJ0IGluIHRoZSBtYXRyaXguIFdoZXJlIGNvdWxkIHRoZXkgZ28gdG8gY3JlYXRlIGEgY29uY2VudHJhdGlvbiBncmFkaWVudCBmb3Igc3ludGhlc2l6aW5nIEFUUD8=
Cg==[Qq]
[q]In the diagram below, which number represents the area that protons flow to as they flow through ATP synthase, making ATP?
[textentry single_char=”true”]
[c]NQ ==[Qq]
[f]IFllcy4g4oCcNeKAnSBpcyB0aGUgbWF0cml4LCB3aGljaCBpcyB3aGVyZSBwcm90b25zIGZsb3cgYXMgdGhleSBmbG93IGZyb20gdGhlIGludGVybWVtYnJhbmUgc3BhY2UsIHRocm91Z2ggQVRQIHN5bnRoYXNlLCBpbnRvIHRoZSBtYXRyaXgu[Qq]
[c]Kg==[Qq]
[f]Tm8uIEhlcmUmIzgyMTc7cyBhIGhpbnQuIFRoZSBwcm90b25zIGhhdmUgYmVlbiBwdW1wZWQgdG8gdGhlIGludGVybWVtYnJhbmUgc3BhY2UuIFRoZSBvdXRlciBtZW1icmFuZSBpcyBpbXBlcm1lYWJsZSB0byBwcm90b25zLiBTbyBpcyB0aGUgaW5uZXIgbWVtYnJhbmUsIGV4Y2VwdCBmb3IgdGhlIEFUUCBzeW50aGFzZSBjaGFubmVsLiBJZiB0aGUgcHJvdG9ucyBmbG93IHRocm91Z2ggQVRQIHN5bnRoYXNlLCB3aGVyZSBhcmUgdGhleSBnb2luZyB0byB3aW5kIHVwPw==
Cg==[Qq]
[q]In the diagram below, where is NADH oxidized?
[textentry single_char=”true”]
[c]Ng ==[Qq]
[f]IFllcy4g4oCcNuKAnSBpcyB0aGUgaW5uZXIgbWVtYnJhbmUsIHdoaWNoIGlzIHdoZXJlIE5BREggaXMgb3hpZGl6ZWQsIGdpdmluZyB1cCBpdHMgZWxlY3Ryb25zIHRvIHRoZSBlbGVjdHJvbiB0cmFuc3BvcnQgY2hhaW4u[Qq]
[c]Kg==[Qq]
[f]Tm8uIEhlcmUmIzgyMTc7cyBhIGhpbnQuIFdoZW4gTkFESCBpcyBveGlkaXplZCwgaXQgZ2l2ZXMgdXAgZW5lcmdldGljIGVsZWN0cm9ucyB0aGF0IGZsb3cgYWxvbmcgdGhlIGVsZWN0cm9uIHRyYW5zcG9ydCBjaGFpbi4gV2hlcmUgaXMgdGhlIGVsZWN0cm9uIHRyYW5zcG9ydCBjaGFpbiBsb2NhdGVkPw==[Qq]
[q]Within the mitochondria, where is NAD+ reduced to NADH?
[textentry single_char=”true”]
[c]NQ ==[Qq]
[f]IFllcy4g4oCcNeKAnSBpcyB0aGUgbWF0cml4LiBEdXJpbmcgYm90aCB0aGUgbGluayByZWFjdGlvbiBhbmQgdGhlIEtyZWJzIGN5Y2xlLCBOQUQ=Kw==IGdldHMgcmVkdWNlZCB0byBOQURILCBhbmQgYm90aCB0aGVzZSBwcm9jZXNzZXMgb2NjdXIgaW4gdGhlIG1pdG9jaG9uZHJpYWwgbWF0cml4Lg==[Qq]
[c]Kg==[Qq]
[f]Tm8uIEhlcmUmIzgyMTc7cyBhIGhpbnQuIFR3byBwcm9jZXNzZXMgd2l0aGluIHRoZSBtaXRvY2hvbmRyaWEgcmVkdWNlIE5BRA==Kw==IHRvIE5BREguIFRoZXNlIGFyZSB0aGUgbGluayByZWFjdGlvbiBhbmQgdGhlIEtyZWJzIGN5Y2xlLiBXaGVyZSB3aXRoaW4gdGhlIG1pdG9jaG9uZHJpYSBkbyB0aGVzZSBwcm9jZXNzZXMgb2NjdXI/
Cg==[Qq]
[q]Which number or letter below represents the electron transport chain?
[textentry single_char=”true”]
[c]Mg ==[Qq]
[f]IFllcy4g4oCcMuKAnSByZXByZXNlbnRzIHRoZSBlbGVjdHJvbiB0cmFuc3BvcnQgY2hhaW4u[Qq]
[c]Kg==[Qq]
[f]Tm8uIEhlcmUmIzgyMTc7cyBhIGhpbnQuIEVsZWN0cm9ucyBhcmUgZG9uYXRlZCBieSBOQURIIGFuZCBGQURIMg==LiBXaGljaCBwYXJ0IG9mIHRoZSBkaWFncmFtIGNvdWxkIHJlcHJlc2VudCB0aGUgZmxvdyBvZiB0aGVzZSBlbGVjdHJvbnMgdGhyb3VnaCB0aGUgaW5uZXIgbWl0b2Nob25kcmlhbCBtZW1icmFuZT8=
Cg==[Qq]
[q]Which number or letter below shows active transport?
[textentry single_char=”true”]
[c]Mw ==[Qq]
[f]IFllcy4g4oCcM+KAnSByZXByZXNlbnRzIHRoZSBhY3RpdmUgdHJhbnNwb3J0IG9mIHByb3RvbnMgZnJvbSB0aGUgbWF0cml4ICh3aGVyZSB0aGV5JiM4MjE3O3JlIGluIGxvd2VyIGNvbmNlbnRyYXRpb24pIHRvIHRoZSBpbnRlcm1lbWJyYW5lIHNwYWNlICh3aGVyZSB0aGV5JiM4MjE3O3JlIGluIGhpZ2hlciBjb25jZW50cmF0aW9uKQ==[Qq]
[c]Kg==[Qq]
[f]Tm8uIEhlcmUmIzgyMTc7cyBhIGhpbnQuIEFjdGl2ZSB0cmFuc3BvcnQgaXMgdXAgYSBjb25jZW50cmF0aW9uIGdyYWRpZW50LiBFeGFtaW5lIHRoZSBtYXRyaXggYW5kIHRoZSBpbnRlcm1lbWJyYW5lIHNwYWNlLiBXaGVyZSBkbyB5b3Ugc2VlIHNvbWV0aGluZyBnZXR0aW5nIHB1bXBlZCBmcm9tIGxvd2VyIGNvbmNlbnRyYXRpb24gdG8gaGlnaGVyIGNvbmNlbnRyYXRpb24/
Cg==[Qq]
[q]Which number or letter below shows ATP synthase?
[textentry single_char=”true”]
[c]Ng ==[Qq]
[f]IFllcy4g4oCcNuKAnSByZXByZXNlbnRzIEFUUCBzeW50aGFzZS4=[Qq]
[c]Kg==[Qq]
[f]Tm8uIEhlcmUmIzgyMTc7cyBhIGhpbnQuIEFUUCBzeW50aGFzZSBpcyBib3RoIGFuIGVuenltZSB0aGF0IG1ha2VzIEFUUCBhbmQgYSBwcm90b24gY2hhbm5lbC4gV2hpY2ggcGFydCBvZiB0aGUgZGlhZ3JhbSBjb3VsZCBiZSBib3RoIG9mIHRoZXNlIHRoaW5ncz8=
Cg==[Qq]
[q]Which letter or number represents a region that would have the lowest pH?
[textentry single_char=”true”]
[c]Qw ==[Qq]
[f]IFllcy4g4oCcQ+KAnSBpcyB0aGUgaW50ZXJtZW1icmFuZSBzcGFjZS4gVGhlIGFjY3VtdWxhdGlvbiBvZiBwcm90b25zIGluIHRoaXMgYXJlYSB3b3VsZCBnaXZlIGl0IHRoZSBsb3dlc3QgcEggb2YgYW55IGFyZWEgd2l0aGluIHRoZSBtaXRvY2hvbmRyaW9uLg==[Qq]
[c]Kg==[Qq]
[f]Tm8uIEhlcmUmIzgyMTc7cyBhIGhpbnQuIHBIIGlzIGEgbWVhc3VyZSBvZiBoeWRyb2dlbiBpb24gY29uY2VudHJhdGlvbi4gV2hpY2ggcmVnaW9uIGhhcyBhIGxvdCBvZiBoeWRyb2dlbiBpb25zIChvciBwcm90b25zKSwgYW5kIHdvdWxkIHRoZXJlZm9yZSBoYXZlIGEgbG93ZXJlZCBwSD8=
Cg==[Qq]
[q]Which letter or number best represents “proton-motive force?”
[textentry single_char=”true”]
[c]Ng ==[Qq]
[f]IFllcy4g4oCcNuKAnSBzaG93cyBwcm90b25zIGRpZmZ1c2luZyBmcm9tIHRoZSBpbnRlcm1lbWJyYW5lIHNwYWNlLCB0aHJvdWdoIEFUUCBzeW50aGFzZSwgaW50byB0aGUgbWF0cml4LiBUaGlzIGRpZmZ1c2lvbiBkcml2ZXMgdGhlIHN5bnRoZXNpcyBvZiBBVFAgZnJvbSBBRFAgYW5kIFBpLCBhbmQgdGhlIGZvcmNlIHRoYXQgbWFrZXMgdGhpcyBwb3NzaWJsZSBpcyAmIzgyMjA7cHJvdG9uIG1vdGl2ZSBmb3JjZS4mIzgyMjE7[Qq]
[c]Kg==[Qq]
[f]Tm8uIEhlcmUmIzgyMTc7cyBhIGhpbnQuIFByb3Rvbi1tb3RpdmUgZm9yY2UgaXMgdGhlIGZvcmNlIHRoYXQgZHJpdmVzIEFUUCBzeW50aGVzaXMgYXMgcHJvdG9ucyBkaWZmdXNlIGZyb20gdGhlIGludGVybWVtYnJhbmUgc3BhY2UgaW50byB0aGUgbWF0cml4LiBXaGljaCBudW1iZXIgc2hvd3MgdGhpcyBoYXBwZW5pbmc/
Cg==[Qq]
[q]Which letter or number shows a process that does not involve pumping and which lowers the proton concentration in the matrix?
[textentry single_char=”true”]
[c]NQ ==[Qq]
[f]IFllcy4g4oCcNeKAnSBzaG93cyBveHlnZW4tYWJzb3JiaW5nIGVsZWN0cm9ucyBmcm9tIHRoZSBlbGVjdHJvbiB0cmFuc3BvcnQgY2hhaW4gYW5kIHByb3RvbnMgKEg=Kw==KSBmcm9tIHRoZSBtYXRyaXguIFRoaXMgbG93ZXJzIHRoZSBwcm90b24gY29uY2VudHJhdGlvbiBpbiB0aGUgbWF0cml4LCBpbmNyZWFzaW5nIHRoZSBjb25jZW50cmF0aW9uIGdyYWRpZW50IHRoYXQgZHJpdmVzIEFUUCBzeW50aGVzaXMgYXMgcHJvdG9ucyBkaWZmdXNlIHRocm91Z2ggdGhlIEFUUCBzeW50aGFzZS4=[Qq]
[c]Kg==[Qq]
[f]Tm8uIExvb2sgY2xvc2VseSBhdCB0aGUgZGlhZ3JhbS4gVGhlcmUmIzgyMTc7cyBvbmx5IG9uZSBwbGFjZSB3aGVyZSBwcm90b25zIGFyZSBiZWluZyBhYnNvcmJlZCBieSB0aGUgbWF0cml4LCBhbmQgaXQmIzgyMTc7cyBhdCB0aGUgdmVyeSBlbmQgb2YgdGhlIGVsZWN0cm9uIHRyYW5zcG9ydCBjaGFpbiAoaXQmIzgyMTc7cyBub3QgJiM4MjIwOzMsJiM4MjIxOyB3aGljaCBpbnZvbHZlcyBwdW1waW5nKS4=
Cg==[Qq]
[q]At numbers 1 and 4, mobile electron carriers are being
[c]b3hpZG l6ZWQg[Qq][c]cmVkdWNlZCA=[Qq][c]cGhvc3Bob3J5bGF0ZWQ=[Qq]
[f]IFllcy4gQXMgTkFESCBhbmQgRkFESA==Mg==IGJyaW5nIGVsZWN0cm9ucyB0byB0aGUgZWxlY3Ryb24gdHJhbnNwb3J0IGNoYWluLCB0aGV5IGJlY29tZSBveGlkaXplZCB0byBOQUQ=Kw==IGFuZCBGQUQu[Qq]
[f]Tm8uIFJlZHVjdGlvbiBpcyB0aGUgZ2FpbiBvZiBlbGVjdHJvbnMuIEZvciBleGFtcGxlLCB3aGVuIE5BRA==Kw==IGJlY29tZXMgTkFESCwgdGhhdCYjODIxNztzIGEgcmVkdWN0aW9uLg==[Qq]
[f]Tm8uIFBob3NwaG9yeWxhdGlvbiBpcyB0aGUgZ2FpbiBvZiBhIHBob3NwaGF0ZSBncm91cC4gVGhhdCYjODIxNztzIG5vdCB3aGF0JiM4MjE3O3MgaGFwcGVuaW5nIGhlcmUu
Cg==[Qq]
[q]What’s happening at “6” in the diagram below is
[c]b3hpZGF0aW9u[Qq]
[c]cmVkdWN0aW9u[Qq]
[c]c3Vic3RyYXRlIGxldmVsIHBob3NwaG9yeWxhdGlvbg==[Qq]
[c]b3hpZGF0aXZlIHBob3 NwaG9yeWxhdGlvbg==[Qq]
[f]Tm8uIE94aWRhdGlvbiBpcyB0aGUgbG9zcyBvZiBlbGVjdHJvbnMuIExvb2sgY2xvc2VseSBhdCB3aGF0JiM4MjE3O3MgaGFwcGVuaW5nIGF0ICYjODIyMDs2LiYjODIyMTsgTm90aWNlIHRoYXQgaXQgaW52b2x2ZXMgYSBwaG9zcGhhdGUgKHdoaWNoIGlzIGEgYmlnIGhpbnQpLg==[Qq]
[f]Tm8uIFJlZHVjdGlvbiBpcyB0aGUgZ2FpbiBvZiBlbGVjdHJvbnMuIExvb2sgY2xvc2VseSBhdCB3aGF0JiM4MjE3O3MgaGFwcGVuaW5nIGF0ICYjODIyMDs2LiYjODIyMTsgTm90aWNlIHRoYXQgaXQgaW52b2x2ZXMgYSBwaG9zcGhhdGUgKHdoaWNoIGlzIGEgYmlnIGhpbnQpLg==[Qq]
[f]Tm8uIFN1YnN0cmF0ZSBsZXZlbCBwaG9zcGhvcnlsYXRpb24gaW52b2x2ZXMgZW56eW1lcyB0aGF0IHRyYW5zZmVyIGEgcGhvc3BoYXRlIGZyb20gYW4gb3JnYW5pYyBzdWJzdHJhdGUgYW5kIHBsYWNlIGl0IG9udG8gQURQLCBtYWtpbmcgQVRQLiAmIzgyMjA7NiYjODIyMTsgaXMgcGhvc3Bob3J5bGF0aW9uLCBidXQgaXQmIzgyMTc7cyBub3QgJiM4MjIwO3N1YnN0cmF0ZS1sZXZlbCYjODIyMTs=[Qq]
[f]WWVzLiBUaGUgcGhvc3Bob3J5bGF0aW9uIHRoYXQgaGFwcGVucyBhdCBBVFAgc3ludGhhc2UgaXMgY2FsbGVkICYjODIyMDtveGlkYXRpdmUgcGhvc3Bob3J5bGF0aW9uLCYjODIyMTsgYmVjYXVzZSBpdCByZXF1aXJlcyB0aGUgcHJlc2VuY2Ugb2Ygb3h5Z2VuIHRvIHBvd2VyIHRoZSBlbGVjdHJvbiB0cmFuc3BvcnQgY2hhaW4u[Qq]
[q]Which letter or number shows where substrate-level phosphorylations are occurring within the mitochondria?
[textentry single_char=”true”]
[c]RQ ==[Qq]
[f]IFllcy4g4oCcReKAnSBpcyB0aGUgbWF0cml4LiBXaXRoaW4gdGhlIG1hdHJpeCwgdGhlIEtyZWJzIGN5Y2xlIGNhcnJpZXMgb3V0IG9uZSBzdWJzdHJhdGUgcGhvc3Bob3J5bGF0aW9uIGZvciBlYWNoIGFjZXR5bCBncm91cCB0aGF0IGlzIGJyb3VnaHQgaW50byB0aGUgY3ljbGUu[Qq]
[c]Kg==[Qq]
[f]Tm8uIEhlcmUmIzgyMTc7cyBhIGhpbnQuIFN1YnN0cmF0ZSBsZXZlbCBwaG9zcGhvcnlsYXRpb25zIG9jY3VyIGR1cmluZyB0aGUgS3JlYnMgY3ljbGUuIFdoZXJlIGRvZXMgdGhlIEtyZWJzIGN5Y2xlIG9jY3VyPw==[Qq]
[q]Of the following protein complexes, which must have the most electronegative electron carriers?
[c]SSA=[Qq][c]SUkg[Qq][c]SUlJIA==[Qq][c]SV Y=[Qq]
[f]IE5vLiBUaGUgZWxlY3Ryb24gY2FycmllcnMgaW4gdGhlIGVsZWN0cm9uIHRyYW5zcG9ydCBjaGFpbiBhcmUgb3JnYW5pemVkIGluIG9yZGVyIG9mIGluY3JlYXNpbmcgZWxlY3Ryb25lZ2F0aXZpdHkuIENvbXBsZXggSSBpcyB0aGUgbGVhc3Q=IGVsZWN0cm9uZWdhdGl2ZS4=[Qq]
[f]IE5vLiBUaGUgZWxlY3Ryb24gY2FycmllcnMgaW4gdGhlIGVsZWN0cm9uIHRyYW5zcG9ydCBjaGFpbiBhcmUgb3JnYW5pemVkIGluIG9yZGVyIG9mIGluY3JlYXNpbmcgZWxlY3Ryb25lZ2F0aXZpdHkuIENvbXBsZXggSSBpcyB0aGUgbGVhc3Q=IGVsZWN0cm9uZWdhdGl2ZS4gV2hpY2ggb25lIHdvdWxkIGJlIHRoZSBtb3N0Pw==[Qq]
[f]IE5vLiBUaGUgZWxlY3Ryb24gY2FycmllcnMgaW4gdGhlIGVsZWN0cm9uIHRyYW5zcG9ydCBjaGFpbiBhcmUgb3JnYW5pemVkIGluIG9yZGVyIG9mIGluY3JlYXNpbmcgZWxlY3Ryb25lZ2F0aXZpdHkuIENvbXBsZXggSSBpcyB0aGUgbGVhc3Q=IGVsZWN0cm9uZWdhdGl2ZS4gV2hpY2ggb25lIHdvdWxkIGJlIHRoZSBtb3N0Pw==[Qq]
[f]IFllcy4gVGhlIGVsZWN0cm9uIGNhcnJpZXJzIGluIHRoZSBlbGVjdHJvbiB0cmFuc3BvcnQgY2hhaW4gYXJlIG9yZ2FuaXplZCBpbiBvcmRlciBvZiBpbmNyZWFzaW5nIGVsZWN0cm9uZWdhdGl2aXR5LiBDb21wbGV4IEkgaXMgdGhlIA==bGVhc3Q=IGVsZWN0cm9uZWdhdGl2ZSwgYW5kIGNvbXBsZXggSVYgaXMgdGhlIG1vc3QgZWxlY3Ryb25lZ2F0aXZlLg==
[/qwiz]
6. Electron Transport Chain: Interactive Lyrics
Working with the lyrics is a great way to learn the details of the electron transport chain and chemiosmosis.
[qwiz qrecord_id=”sciencemusicvideosMeister1961-Electron Transport Chain Fill-in-the-Blanks (M10)”]
[h]The Electron Transport Chain Song: Interactive Lyrics
[i]
[q labels = “top”]
Welcome to this story about cell _______.
The goal is explaining how cells make ATP
It happens in the ____________ which you can think of
As the cell’s energy factory
Mitochondria are double-membraned organelles,
An inner membrane and an outer one as well
The mitochondrial _______ is the fluid inside
It’s where reactions like ______ cycle reside
[l]energy
[fx] No, that’s not correct. Please try again.
[f*] Correct!
[l]mitochondria
[fx] No. Please try again.
[f*] Good!
[l]matrix
[fx] No, that’s not correct. Please try again.
[f*] Excellent!
[l]Krebs
[fx] No, that’s not correct. Please try again.
[f*] Excellent!
[q labels = “top”]
__________ and Krebs make NADH
and FADH2 from energy in food
These ________ carriers make their way
From the matrix to the inner membrane.
And that’s where you find the electron transport chain
It’s a series of _________ embedded in the membrane,
Which take the electron carrier’s electrons
And uses their energy for pumping ________
[l]electron
[fx] No. Please try again.
[f*] Excellent!
[l]enzymes
[fx] No. Please try again.
[f*] Good!
[l]glycolysis
[fx] No. Please try again.
[f*] Excellent!
[l]protons
[fx] No, that’s not correct. Please try again.
[f*] Good!
[q labels = “top”]
The mitochondrial electron transport chain
Uses electron energy for pumping protons
From the mitochondrial ________ to the intermembrane space
Increasing proton concentration in that place,
The only way the protons can escape
Is through a channel and an enzyme, ATP __________.
Which uses diffusing protons’ kinetic energy
To make ATP, from ______ and P
[l]ADP
[fx] No, that’s not correct. Please try again.
[f*] Correct!
[l]matrix
[fx] No. Please try again.
[f*] Great!
[l]synthase
[fx] No, that’s not correct. Please try again.
[f*] Excellent!
[q labels = “top”]
The chain is a series of _________ in a row
Each accepts __________, then lets them go
To the next carrier in this transport chain.
It’s kind of organized like a bucket brigade
What drives electrons down this enzymatic series
Is the growing level of each carrier’s _______________
And to _________ electrons ultimately fall
It’s the most electronegative of them all.
[l]electronegativity
[fx] No, that’s not correct. Please try again.
[f*] Correct!
[l]electrons
[fx] No. Please try again.
[f*] Correct!
[l]enzymes
[fx] No, that’s not correct. Please try again.
[f*] Good!
[l]oxygen
[fx] No. Please try again.
[f*] Excellent!
[q labels = “top”]
It’s NADH that starts this run
Donating electrons to Complex number I
This powers ________ transport as protons are displaced.
And get pumped from the matrix to the _____________ space
From Complex I electrons flow to
___________, also known as “Q”
Which floats through the inner membrane happily
And brings its electrons to __________.
[l]active
[fx] No, that’s not correct. Please try again.
[f*] Great!
[l]Complex III
[fx] No, that’s not correct. Please try again.
[f*] Good!
[l]intermembrane
[fx] No, that’s not correct. Please try again.
[f*] Great!
[l]Ubiquinone
[fx] No. Please try again.
[f*] Great!
[q labels = “top”]
And you can guess the function of Complex III.
It’s another proton _______ using _________ energy
Protons jam up in that intermembrane compartment
Like a hundred people in a one-bedroom apartment!
CHORUS
Complex II is for FADH2
Which donates electrons, which then get passed to ____
Which once again passes them to Complex III
Which pumps _________ using electron energy
[l]electron
[fx] No. Please try again.
[f*] Good!
[l]protons
[fx] No, that’s not correct. Please try again.
[f*] Excellent!
[l]pump
[fx] No. Please try again.
[f*] Great!
[l]Q
[fx] No. Please try again.
[f*] Good!
[q labels = “top”]
From Complex III the electrons proceed
To another mobile carrier, ___________ C
Which donates the electrons to Complex IV
Another proton pump, could you ask for more?
After Complex IV __________ flow,
To oxygen which is ever so
______________ it pulls electrons down the _______,
Keeping them moving like the cars of a train.
[l]chain
[fx] No. Please try again.
[f*] Great!
[l]Cytochrome
[fx] No, that’s not correct. Please try again.
[f*] Great!
[l]electronegative
[fx] No. Please try again.
[f*] Good!
[l]electrons
[fx] No, that’s not correct. Please try again.
[f*] Excellent!
[q labels = “top”]
And as O2 does this electron grabbing trick,
It also grabs protons from the matrix.
They all combine to form ______
Electron __________ chain, watch it go!
CHORUS
Now all these protons in the intermembrane space
Are trapped they can’t get out of that place
‘Cause protons are _________ and could never get through
A ____________ bilayer, they can’t diffuse
[l]charged
[fx] No. Please try again.
[f*] Great!
[l]H2O
[fx] No, that’s not correct. Please try again.
[f*] Good!
[l]phospholipid
[fx] No. Please try again.
[f*] Good!
[l]transport
[fx] No, that’s not correct. Please try again.
[f*] Correct!
[q labels = “top”]
But like all particles, they’re dying to go
From where their concentration’s _______ to where it’s low.
Stuck in the intermembrane space they’re frustrated.
To diffuse to the ________, they’re highly motivated.
And this __________ been made steeper by O2
Which absorbs protons from the matrix stew,
So from proton pumping, and oxygen’s actions.
Add another force, ______________ attraction!
[l]electrochemical
[fx] No, that’s not correct. Please try again.
[f*] Correct!
[l]gradient’s
[fx] No. Please try again.
[f*] Excellent!
[l]high
[fx] No, that’s not correct. Please try again.
[f*] Excellent!
[l]matrix
[fx] No. Please try again.
[f*] Great!
[q labels = “top”]
Think of all those trapped protons, each one’s _________.
The matrix, in comparison, is negative.
Opposites attract, so the protons are dying
To get to the matrix, oh how they’re trying!
There’s only one _________ that lets the protons pass,
And they use it like high school students busting out of class,
It’s a channel and an _________, it’s ATP __________
The closer in this game, an energy ace
[l]channel
[fx] No, that’s not correct. Please try again.
[f*] Great!
[l]enzyme
[fx] No. Please try again.
[f*] Excellent!
[l]positive
[fx] No, that’s not correct. Please try again.
[f*] Great!
[l]synthase
[fx] No. Please try again.
[f*] Correct!
[q labels = “top”]
ATP synthase is embedded in the _______ membrane
How it works is so cool it’s insane.
It’s got channels for diffusing protons running right through it.
When cells make ATP, well watch how they do it
The matrix side of ATP synthase has binding sites
For ADP and _____ which come in and bind.
And as ATP synthase lets protons barge through
Their ________ energy gets put to use.
Like water through a turbine ________ movement generates _________.
Changing synthase’s binding site conformation.
Which catalyzes chemical bond formation.
ADP and P make _______ that energy sensation!
[l]ATP
[fx] No. Please try again.
[f*] Great!
[l]inner
[fx] No. Please try again.
[f*] Good!
[l]kinetic
[fx] No. Please try again.
[f*] Correct!
[l]P
[fx] No, that’s not correct. Please try again.
[f*] Excellent!
[l]proton
[fx] No. Please try again.
[f*] Great!
[l]rotation
[fx] No, that’s not correct. Please try again.
[f*] Correct!
[x]
[restart]
[/qwiz]
7. Electron Transport Chain Fill-in-the-Blanks Quiz
[qwiz random = “true” qrecord_id=”sciencemusicvideosMeister1961-Electron Transport Chain Interactive Lyrics (M10)”]
[h]Electron Transport Chain Fill-in-the-Blanks
[i]
[q]Most of the cell’s [hangman] is made in the mitochondria.
[c]QVRQ[Qq]
[q]The enzyme-rich fluid inside the mitochondrial inner membrane is the [hangman].
[c]bWF0cml4[Qq]
[q]The mitochondrial electron transport chain uses energy in electrons to pump [hangman] into the intermembrane space.
[c]cHJvdG9ucw==[Qq]
[q]The ETC makes ATP when [hangman] diffuse through the ATP synthase channel from the intermembrane space back to the matrix.
[c]cHJvdG9ucw==[Qq]
[q]When protons diffuse from the intermembrane space to the matrix, their kinetic energy is what drives ATP [hangman]. That’s why the enzyme/channel through which protons diffuse is called [hangman] [hangman].
[c]c3ludGhlc2lz[Qq]
[c]QVRQ[Qq]
[c]c3ludGhhc2U=[Qq]
[q]The last electron acceptor in the mitochondrial electron transport chain is [hangman].
[c]b3h5Z2Vu[Qq]
[q]Enzyme Complexes 1, 3, and 4 are all [hangman] pumps.
[c]cHJvdG9u[Qq]
[q]The electrons dropped off by [hangman] have less energy than those dropped off by NADH, and thereby power the pumping of fewer protons (note: don’t worry about typing subscripts)
[c]RkFESDI=[Qq]
[q]As [hangman] accepts electrons from the ETC and soaks up protons from the matrix, it creates water.
[c]b3h5Z2Vu[Qq]
[q]The protons in the intermembrane space can’t diffuse through the [hangman] in the inner membrane.
[c]cGhvc3Bob2xpcGlkcw==[Qq]
[q]Protons in the [hangman] [hangman] “want” to diffuse back to the matrix. But because of the [hangman] bilayer, the only way protons can get from the intermembrane space to the matrix is through the [hangman] [hangman] channel.
[c]aW50ZXJtZW1icmFuZQ==[Qq]
[c]c3BhY2U=[Qq]
[c]cGhvc3Bob2xpcGlk[Qq]
[c]QVRQ[Qq]
[c]c3ludGhhc2U=[Qq]
[q]During ATP synthesis, [hangman] and Pi (inorganic phosphate) bind at binding sites on the matrix side of ATP synthase.
[c]QURQ[Qq]
[q]NADH and FADH2 are [hangman] [hangman].
[c]ZWxlY3Ryb24=[Qq]
[c]Y2FycmllcnM=[Qq]
[q]The mitochondrial electron transport chain is a series of enzymes embedded in the [hangman] mitochondrial membrane.
[c]aW5uZXI=[Qq]
[q]When protons diffuse from the [hangman] space to the [hangman], their kinetic energy is what drives ATP synthesis.
[c]aW50ZXJtZW1icmFuZQ==[Qq]
[c]bWF0cml4[Qq]
[q]In the electron transport chain, each electron carrier is more [hangman] than the one that preceded it.
[c]ZWxlY3Ryb25lZ2F0aXZl[Qq]
[q]In the mitochondrial electron transport chain, proton pumps in the inner mitochondrial membrane pump protons from the [hangman] to the [hangman] space
[c]bWF0cml4[Qq]
[c]aW50ZXJtZW1icmFuZQ==[Qq]
[q]The electrons dropped off by FADH2 have less energy than those dropped off by [hangman], and thereby power the pumping of fewer [hangman].
[c]TkFESA==[Qq]
[c]cHJvdG9ucw==[Qq]
[q]During ATP synthesis, diffusing [hangman] provide kinetic energy to transform [hangman] and Pi into [hangman].
[c]cHJvdG9ucw==[Qq]
[c]QURQ[Qq]
[c]QVRQ[Qq]
[q]Mitochondria have a [hangman] membrane.
[c]ZG91Ymxl[Qq]
[q][hangman] pumping by the ETC creates an electrochemical [hangman] in which protons are much more concentrated in the intermembrane space than they are in the [hangman]. Subsequently, kinetic energy from [hangman] protons catalyzes chemical bond formation to create ATP from ADP and Pi.
[c]cHJvdG9u[Qq]
[c]Z3JhZGllbnQ=[Qq]
[c]bWF0cml4[Qq]
[c]ZGlmZnVzaW5n[Qq]
[x]
[restart]
[/qwiz]
What’s next
- Anaerobic Respiration/Fermentation Tutorial (the next tutorial in this series)
- Cellular Respiration Main Menu
- Cellular Respiration Musical Review

