In the previous unit, we saw how by trading electrons, atoms can become charged ions that bind together through ionic bonds. In this unit, you’ll learn about the most important chemical bond in the molecules that make up living things–the covalent bond.
1. In covalent bonds, atoms share electrons
1.1. Covalent bonding in hydrogen
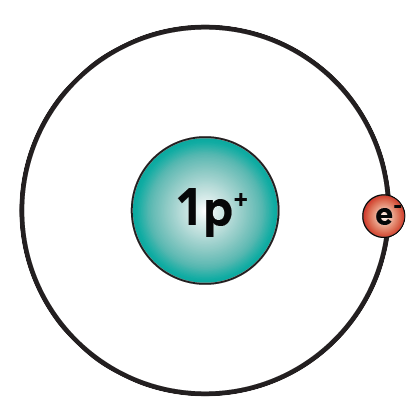
To learn about covalent bonds, let’s start by looking at the structure of a molecule of hydrogen gas, H2. This gas is one of the most abundant substances in the universe.
A hydrogen atom has one proton and one electron.
As a single atom, hydrogen is “unhappy.” Why? Because the first energy level has a capacity of two electrons, and hydrogen only has one. With one electron, hydrogen is “unfulfilled.”
By sharing its electrons with another hydrogen atom, hydrogen can achieve a happier state. Here’s what it looks like when two hydrogen atoms share their two electrons.
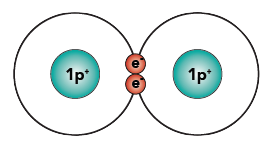
I’ve drawn the two electrons in the middle, between the two nuclei (plural of nucleus). However, you should imagine those two electrons freely orbiting around both of the nuclei. When you imagine it that way, you can see how each nucleus of the hydrogen atoms now has two electrons orbiting around it. As a result, each atom is “happy.” The shared pair of electrons binds the two atoms together. This bond, formed by sharing a pair of electrons, is called a covalent bond.
1.2. Covalent bonding in Methane, CH4.
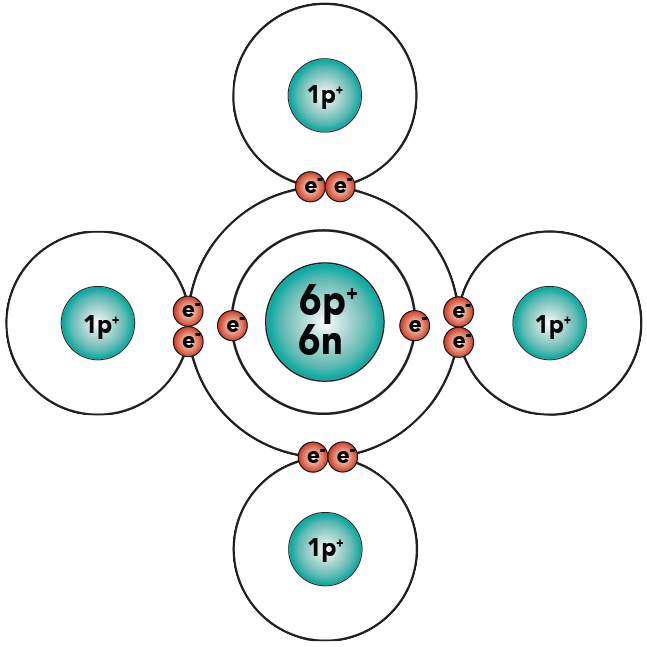
Let’s look at a slightly more complex molecule. The molecule is methane (a common fuel), and its formula is CH4.
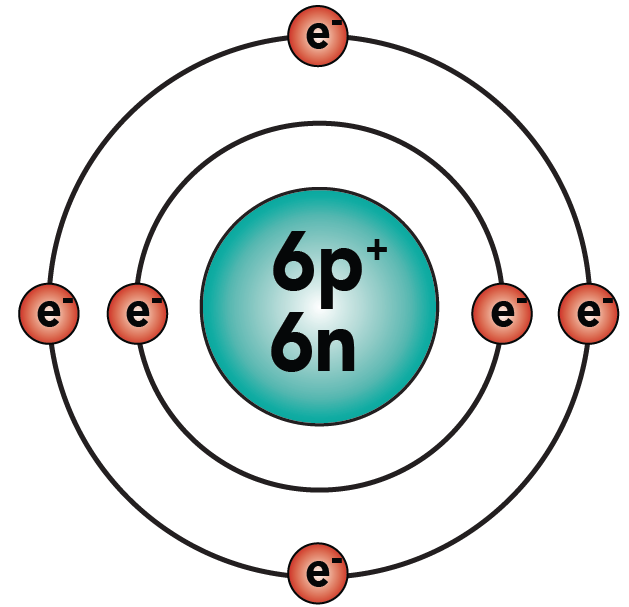
You know that carbon has 6 protons, 6 neutrons, and 6 electrons. Hydrogen, as shown above, has one proton and one electron. Here are the two atoms.
 |
 |
| Carbon: an unhappy atom | Hydrogen: an unhappy atom |
[qwiz style = “width: 528px; min-height:0px; border: 2px solid black; ” qrecord_id=”sciencemusicvideosMeister1961-Covalent bonds, predict methane”]
[h]Predict the Structure of Methane
[q]Knowing that the chemical formula of methane is CH4, can you figure out how to draw methane’s structural formula? There will be one carbon sharing electrons with four hydrogens. Give it a try, then click below to see if you got it right.
[c]c2hvdyB0aG UgYW5zd2Vy[Qq]
[f]IEFOU1dFUg==
Cg==[x]
[/qwiz]
Make sure you’ve clicked “show the answer” and looked at the diagram of methane before going on.
Examine methane, and count the electrons in the carbon atom’s outer energy level. Because of the shared electrons, carbon now has eight electrons in its outer energy level, making it “happy.” And because each hydrogen has two electrons in its outer shell, all of the hydrogens are “happy.”
The atoms connected by covalent bonds make up a covalently bonded molecule. So far, we’ve met two such molecules: hydrogen and methane.
2. Structural Formulas, and a few more examples.
We can represent covalently bonded molecules with a convenient shorthand called a structural formula. There are two rules for drawing them.
- Instead of drawing the atom, just use the chemical symbol.
- Let a dash represent a shared pair of electrons.
Here’s a structural formula for a hydrogen molecule. I’ve also put a diagram showing electron energy levels on the right side so you can compare them.

And here’s a structural formula of methane, with a Bohr model for comparison.
[qwiz style = “width: 528px; min-height: 0px; border: 1px solid black; ” qrecord_id=”sciencemusicvideosMeister1961-Covalent Bonds, predict ethane”]
[h] Apply!
[q] If you’re getting this, you should be able to do the following: Draw the structural formula for C2H6 (ethane). Then click below to see if you got it right.
[c]wqBzaG93IHRo ZSBhbnN3ZXI=[Qq]
[f]IEFOU1dFUg==
Cg==[x]
[/qwiz]
3. Single, Double, and Triple Covalent Bonds
The two molecules we’ve discussed so far, hydrogen and methane, are held together by single covalent bonds. In the diagrams below, each dash represents a shared pair of electrons.
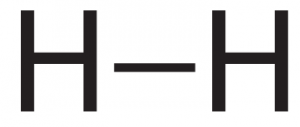 |
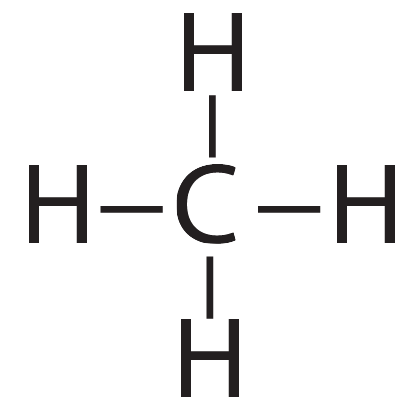 |
| Hydrogen (H2). The two hydrogens are connected by a single bond. | Methane (CH4). The carbon is connected to each hydrogen by a single bond |
Sometimes, molecules are held together by double bonds. A double bond involves two shared pairs of electrons. You can see this in the molecule ethylene, C2H4.
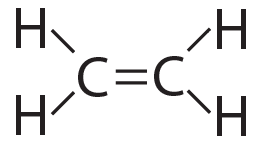 |
 |
| Ethylene (C2H4), structural formula | Ethylene (C2H4), showing electron energy levels |
The carbon atoms are sharing two pairs of electrons. At the same time, each carbon is sharing a single pair of electrons with each hydrogen. The result: four “happy” atoms (each with a complete outer energy level).
Oxygen is another double-bonded molecule.
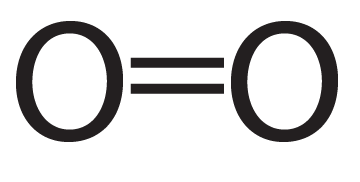 |
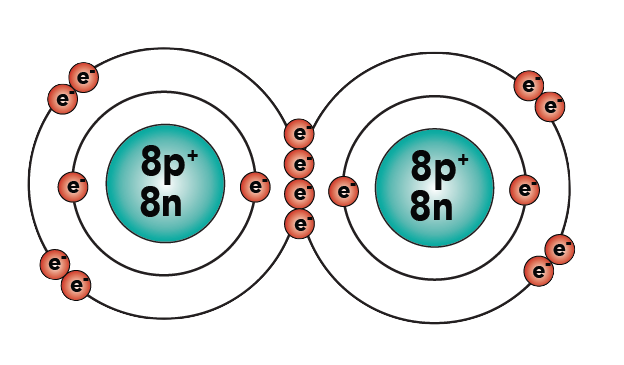 |
| Oxygen (O2), structural formula | Oxygen (O2), showing electron shells |
As you can see, each oxygen now has eight electrons in its outer energy level, making it “happy.”
Even triple bonds are possible.
Study the diagram below.
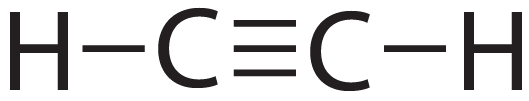
In this compound, C2H2 (Acetylene), the carbons are connected to the hydrogen atoms through single bonds. Triple bonds (three shared pairs of electrons) connect the two carbons.
4. Three Key Ideas
Here’s what you should have learned so far about covalent bonds and structural formulas.
- In a covalent bond, atoms share electrons
- The atoms in a covalently bonded molecule can be held together by
- single bonds (a single shared pair of electrons),
- double bonds (two pairs of electrons), or even
- triple bonds (three pairs of electrons)
- In a structural formula, the element is represented by its chemical symbol
- Single bonds are represented by a dash
- Double bonds are represented by a double dash
- Triple bonds are represented by a triple dash.
5. Checking Understanding
To finish this module on covalent bonds, work with the flashcards below. Just to make sure you haven’t forgotten some key concepts from the previous modules in this unit, I’m including some questions about ionic bonds and other chemical concepts that we’ve covered, too.
[qdeck style=”border: 2px solid black; ” qrecord_id=”sciencemusicvideosMeister1961-covalent bonding flashcards (M3)”]
[h] Flashcards: Covalent Bonding
[i] Welcome to this set of flashcards about covalent bonds and structural formulas.
- Click ‘Check Answer’ to see the answer to each card.
- If you know it, click ‘Got it.”
- If you don’t know it as well as you’d like, click ‘Need more practice,’ and that card will go to the bottom of the deck so you can practice it again.
- ‘Shuffle’ lets you shuffle the deck.
[start]
[q] The type of chemical bond where atoms share electrons is called a(n) ________ bond.
[textentry]
[a] The type of chemical bond where atoms share electrons is called a covalent bond
[q] The type of chemical bond where atoms trade electrons is called a (n) _______ bond.
[textentry]
[a] The type of chemical bond where atoms trade electrons is called an ionic bond.
[q] The diagram below is a _________ formula.
[textentry]
[a] This type of diagram is a structural formula
[q] Is “C2H2” a structural formula or a chemical formula?
[textentry]
[a] A formula like “C2H2,” which just shows the type of atoms and the number of atoms, is a chemical formula
[q] In the molecule shown below, the type of covalent bond between the two hydrogen atoms is a ________ bond.
[textentry]
[a] In the molecule shown below, the type of covalent bond between the two hydrogen atoms is a single bond.
[q] In the molecule shown below, the type of covalent bond between the two nitrogen atoms is a __________ bond.
[textentry]
[a] In the molecule shown below, the type of covalent bond between the two nitrogen atoms is a triple bond
[q] In the molecule below, the type of covalent bond indicated by the red arrow is a ________ bond.
[textentry]
[a] In the molecule below, the type of covalent bond indicated by the red arrow is a double bond.
[q] In the formula C12H22O11, how many oxygen atoms are present?
[textentry]
[a] In the formula C12H22O11, there are 11 oxygen atoms.
[x]
If you want more practice, please press the restart button below. Otherwise, continue to the final quiz.
[restart]
[/qdeck]
Next steps
It’s impossible to overstate the importance of covalent bonds in biology. An entire discipline – biochemistry – focuses on the structure and function of covalently bonded molecules in living things. We’ll explore these molecules later in this course.
If you need more practice related to understanding covalent bonds, please scroll up to the top and work through this tutorial again. Otherwise, please continue to Basic Chemistry Conclusion and Final Quiz